International Journal of Advancements in Technology
Open Access
ISSN: 0976-4860
+44 1478 350008
ISSN: 0976-4860
+44 1478 350008
Research Article - (2017) Volume 8, Issue 4
Nanoparticles possess unique properties, which can be applied in medical applications; they address targets such as cellular therapy, tissue repair, Nano-biosensors, drug delivery, magnetic resonance imaging and magnetic fluid hyperthermia. In this work, different sized cobalt ferrite nanoparticles (CFNP) were synthesized with narrow size distribution, by using chemical precipitation methods, to aim for finding the optimum particle size which has high heating efficiency in the applied magnetic field. The obtained powder was calcined at different temperature (600°C, 800°C, 900°C, and 1000°C). The sample which characterized by Transmission Electron Microscopy (TEM) confirmed the formation of single-phase CFNP in the range 10–115 nm depending on the annealing temperature and Vibrating Sample Magnetometer (VSM) to get the magnetization and coercivity of the powder. Localized magnetic particle hyperthermia treatment using ferrimagnetic nanoparticles continue to be an active area of medical application. So, homemade induction heater was designed. The induction heater was designed to be capable of generating high frequency, strong alternative magnetic fields (8 kA·m–1, 135 kHz). In vitro heating efficiencies in test tube, at a MNPs concentration of 250 mg CFNP·ml-1, were measured in the applied field. The temperature increase (ΔT) of the tube content at 60 s was 29.9°C for MNPs of 18 nm, 26.7°C for 25 nm, 25°C for 60 nm and 22.9°C for MNPs of 95 nm. The smallest nanoparticles (18 nm) exhibiting a high heating efficiency. In conclusion, we found that the size of the CFNP increased with increasing the calcined temperature at which the synthesis of the nanoparticles was performed. The heating efficiency of the particles was improved with decreasing particle size from 95 nm to 18 nm in the alternating magnetic field.
<Keywords: Cobalt ferrite; Nanoparticles size; Magnetic hyperthermia
Biologic and biomedical applications of Nano scale materials in health care have rapidly progressed; nanomaterials have attracted much attention because of their potential for use as efficient drug delivery carriers for diagnostic and therapeutic applications. Nanoparticles offer some attractive possibilities in biomedicine. First, they have controllable sizes ranging from a few nanometers up to tens of nanometers which places them at size that is smaller than or comparable to those of a human hair 60-120 μm wide, cells 10–100 μm, a virus 20–450 nm, a protein 5–50 nm or a gene (2 nm wide and 10–100 nm long). Magnetic nanoparticles of spinel ferrites are interesting in fundamental science [1]. Magnetic nanoparticles (MNPs) are being of great interest for a wide range of disciplines, such as biomedicine, magnetic fluids, magnetic energy storage, and catalysis [2,3]. In many electronic and magnetic applications, it is important to make a ceramic material of desirable microstructure, with a high sintered density, a small particle size and a narrow particle size distribution [4]. Uses of MNPs in biotechnology and biomedicine have dramatically increased over the last years. They can be grouped into two broader categories depending on the methodology: in vitro and in vivo procedures. For in vitro applications, the main use is in diagnoses and separation/labelling of biomoleculessuch as protein, cell, Deoxyribonucleic Acid (DNA)/ Ribonucleic Acid (RNA), microorganism for in vivo applications can be further split into (i) therapies (drug delivery and hyperthermia) and (ii) diagnoses (Magnetic Resonance Imaging (MRI)) [5]. In the diagnostic medical field, MNPs are used as contrast agents to enhance the contrast in MRI. In tumor therapy where they can be selectively introduced into the tumor cells and then their temperature is increased using an oscillating magnetic field, and finally used as site-specific drug delivery agents which involve immobilizing the drug on magnetic materials under the action of external magnetic field. Ferro-fluids of nanoparticles were used in the treatment of solid tumors and in the diagnosis and treatment of diseases [2]. The healing power of heat has been used to cure a variety of different diseases. This heat treating method which called magnetic hyperthermia was then recognized as the new and promising form of cancer therapy aside from surgery, chemotherapy, and irradiation. Magnetic hyperthermia is a method to induce hyperthermia using MNPs. In this approach MNPs are firstly introduced into the desired tissues and then guided by an external magnetic field, an externally applied oscillating magnetic field induces the hyperthermia [5]. The application of MNPs offers the possibility of a self-limitation of the temperature enhancement by using a magnetic material with suitable Curie temperature, Such MNPs can bind to drugs, enzymes, proteins, nucleotides, or antibodies and can be directed to an organ, tissue, or tumor using an external magnetic field [6,7]. Ferrites are magnetic ceramics of great importance in the production of electronic components since they reduce energy losses which caused by induced currents acting as electric insulators. Their applications range from simple function device, as small permanent magnets to sophisticated devices for the electronic industry [8]. Ferrites were discovered thousands of years ago. The spinel ferrites are very much important magnetic materials due to their combined electrical and magnetic properties, makes ferrite useful in many technological applications. The basic electrical and magnetic properties of ferrite can be modified so as to suit the desired application [9]. The properties of ferrites have been improved through extensive research. In 1960, ferrites attract worldwide attention because new applications such as microwave devices, electronic media, computer and telephone industry were rapidly expanding [10]. They have potential applications in high-density magnetic recording devices especially those with high coercivity in medicine and other electronic devices. One of the most recent applications studied is the complete decomposition of CO2. This decomposition has been significantly improved by developing ultrafine ferrite particles with the high surface area as a catalyst [11]. MNPs being subjected to a magnetic AC field may show remarkable heating effects related to losses during, different processes of magnetization reversal in systems of MNPs. There are various theories which explain the reasons for the heating of the MNPs when subjected to an oscillating electromagnetic field. There exist at least four different mechanisms by which magnetic materials can generate heat in an alternating field, which depend in different manners on the applied magnetics alternative field strength and frequency. The magnetic losses to be utilized for heating arise [6-12]. If a magnetic sample is placed in a liquid of low viscosity and exposed to an alternating field, the sample reacts with oscillating or rotating movements, as a whole (because of the torque exerted on the magnetic moment by the external AC magnetic field), towards the field with the moment locked along the crystal under the effect of a thermal force against a viscous drag in a suspending medium, in order to achieve the position of lowest energy, which lead to losses in the surrounding liquid [12-14]. The physical and chemical properties of spinel nanoparticles are greatly affected by the synthesis route. Therefore, a large number of researcher’s reports are available concerning the preparation techniques. Quality ferrites continued to be prepared to have new properties [12-15]. Among the various methods for producing nanoparticles precipitation methods is highly interesting and have been widely used for their versatility due to their straightforward nature and their potential to produce a large quantity of the final product, low temperature for preparation, relatively simple and providing good control over particle properties. Realizable particle sizes range in size from nanometers to micrometers [16-18]. Most studies suggested that the cobalt ferrites (CoFe2O4) could be promising materials for various applications [19]. CoFe2O4 nanoparticles are of interest because of their unique magnetic, electronic, optical and physical properties such as high Curie temperature, large magnetocrystalline anisotropy, large magnetostrictive coefficient, excellent chemical stability. Metal and alloys unstable under atmospheric conditions, good thermal stability, high electrical resistivity (recording media, memory cores, high-frequency devices) and mechanical hardness for its catalytic properties. This kind of ferrite is a spinel (cubic spinel structure). In addition to the precise control on the composition and structure of CoFe2O4 [10,13,18-21].
CoFe2O4 particle size increase with increasing annealing temperature due to more metal ions are produce which in turn increases the particle sizes, tiny amount of agglomeration is observed at higher temperature due to growing distribution of particle size [6].
Heating of MNP occur by two mechanisms Neel and Brownian relaxation. Neel relaxation occurs when the MNP remains stationary and the moment rotates within the crystal. In the absence of applied magnetic field this leads to the vanishing of the magnetization [14,15,22].
In the Neel mode, the magnetic moment originally locked along the crystal easy axis rotates away from that axis towards the external field. The Neel mechanism is analogous to the hysteresis loss in multi-domain MNPs whereby there is an ‘internal friction’ due to the movement of the magnetic moment in an external field that results in heat generation [23].
Heating can also be due to the rotational Brownian motion within a carrier liquid. If a magnetic sample is placed in a liquid of low viscosity and exposed to an oscillating field the sample reacts with oscillating or rotating movements [24].
Specific absorption rate (SAR) is defined as the amount of heat released by a unit weight of the material per unit time during exposure to an oscillating magnetic field of defined frequency and field strength. It is determined by the “rate of temperature rise” [25].
SAR also denoted specific loss power is defined as the power of heating of a magnetic material per gram.
SAR is measured as: 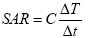
Where C is the specific heat capacity of the sample. It is proportional to the rate of the temperature increase (ΔT/Δt). When nanoparticles are dispersed in a gel or in a liquid, contribution of specific heat capacities of the surrounding media must be taken into account [26].
The heat generated strongly depends on the nature and structure of the particles such as particle size and size distribution, anisotropy constant, saturation magnetization, surface modification, frequency, strength of the applied magnetic field, duration of application of the magnetic field, dispersion of the MNPs within the tissue, and carrier viscosity [27].
Li et al. show that the heating efficiency of the particles was improved with decreasing particle size from 103 nm to 24 nm [28].
Cobalt ferrite nanoparticles (CFNP) were selectively synthesized with narrow size distribution, by using chemical precipitation methods, Cobalt nitrate hexahydrate (Co(NO3)2.6H2O), ferric nitrate nonahydrate (Fe(NO3)3.9H2O) was purchased from Sigma-Aldrich Company and sodium hydroxide (NaOH) was used as the precipitating agents and deionized water as solvent.
In order to obtain 6 gm weight of CoFe2O4 an amount of 7.4 g of Co(NO3)2 was dissolved in 50 ml of distilled H2O. The solution was mixed and stirred at constant temperature (80°C) in shaking water bath; using a stirring rate of 120 rpm for 15 minutes. An amount of 20.6 g of Fe(NO3)3 was dissolved in 50 ml of distilled H2O. The solution was mixed and stirred at constant temperature (80°C) in shaking water bath, using a stirring rate of 120 rpm for 15 minutes. And after that Co(NO3)2 solution was mixed with the Fe(NO3)3 solution at 80°C in a shaking water bath using a stirring rate of 120 rpm for 30 minutes. A 10 g of NaOH was dissolved in 1/4 litre of distilled H2O and then added stepwise to the reaction mixture. When the reaction was completed, precipitated was shown at the bottom of the reaction mixture, and then 4 or 5 drops of oleic acid solution as a coating agent was mixed with the reaction mixture at temperatures 80°C in the weight of all samples were made similar (4 × 1g), The precipitate separated from the solution, and it was washed for several times with distilled H2O and ethyl ether then precipitate was dried at 100°C for 12 hours. The samples were heated at 600°C, 800°C, 900°C, 1000°C respectively for 12 hours. Each of these samples is subdivided into another 4 samples (A, B, C, D) (4 × 250 mg) for TEM characterization and magnetic hyperthermia application.
Transmission electron microscopy (TEM) is the premier tool for understanding the internal microstructure of materials at the nanometer level. It allows obtaining real-space images of materials with resolutions on the order of a few tenths to a few nanometers, TEM used to analysis cobalt ferrite synthesized by co-precipitation in order to determine the shape and average of the particles. Magnetic properties were done by Vibrating sample magnetometer (VSM) at national research center Egypt. The induction heater circuit built and tested in our medical physics laboratory Umm Al-Qura University, the coil has 10 turns, 1.5 cm diameter, length 3 cm with DC current 1.2A, it generate alternating magnetic field 8 kA m-1, 135 kHz (Figure 1A) the magnetic flux density is measured by magnetometer. Suspensions tubes of cobalt ferrite nanoparticles samples in water with the thermal insulator material are holding within the coil as Figure 1B, where we put the same amount of cobalt ferrite (250 mg/ml) which calcination at different temperature (600°C -800°C -900°C -1000°C). The magnetic hyperthermia applied for one minute, and then we measure temperature by digital temperature meter. The ambient temperature was measured before each evaluation.
Transmission electron microscope results
TEM which we used to analyse cobalt ferrite synthesized by coprecipitation, in order to determine the shape and average size of the particles. TEM images display different morphologies and size of cobalt ferrite nanoparticles, where calcination at various temperature (600°C, 800°C, 900°C, and 1000°C).
In Figures 2A and 2B the substance involves dispersion of nearly spherical particles with narrow size distribution, which spurring their natural of the nanoparticle. The size of the particle as shown by TEM is including with mean size distribution of 10 to 25 nm.
Figures 3A and 3B shows the existence of large few agglomerates including hundreds of particles. The mean of particle size is about 15- 35 nm, as evident in this figure sample involves dispersion of nearly spherical particles.
Figures 4A and 4B shows the existence of large agglomerates. The mean of particle size is about 20-100 nm, as evident in this figure sample involves dispersion of nearly spherical particles.
Figures 5A and 5B shows the existence of largest agglomerates including hundreds of particles. The mean of particle size is about 75- 115 nm. There is the majority of particles appears spherical in the shape although some elongated particles are also existence.
In general particle size increment with increment temperature of reaction due to the ratio of reaction is the increment, where these MNPs tend to agglomeration towards each other because of magnetic dipoledipole interaction between particles. In order to achieve better sample to produce hyperthermia, we made an electrical circuit (induction heater) as evident in Figure 1A where we put the same amount of cobalt ferrite which calcination at different temperature (600°C -800°C -900°C -1000°C) and distilled water into four different small tubes. And then we test these small tubes by putting a small tube inside a coil of the electrical circuit to measure temperature by digital temperature meter.
Vibrating sample magnetometer (VSM) results
Vibrating sample magnetometer is used to reorganization magnetic properties of the cobalt ferrite were measured at room temperature. We can determine saturation magnetization, MS, remnant magnetization, Mr and coercivity HC, from obtained hysteresis loops. In Figure 6 the saturation magnetization value of cobalt ferrite MS, where sample calcination at 600°C is measured about 42.38 emu/g and remanace magnetization value MR for this sample are 12.41 emu/g.
Also in Figure 7 we find the saturation magnetization value of cobalt ferrite MS, where sample calcination at 800°C is measured about 26.03 emu/g and remanace magnetization value MR for this sample are 8.33 emu/g. While in the Figure 8 we find the saturation magnetization value of cobalt ferrite MS, where sample calcination at 900°C is measured about 25.6 emu/g and remanace magnetization value MR for this sample are 4.70 emu/g.
In the Figure 9 we find the saturation magnetization value of cobalt ferrite MS, where sample calcination at 1000°C is measured about 18.06 emu/g and remanace magnetization value MR for this sample are 2.45 emu/g.
The size and shape of the hysteresis loops for cobalt ferrite nanoparticles elucidate a kind of thick S-shape. The thickness of the S-shape characterization the amount of hysteresis or the coercivity of the substance where the value of Hc for all sample in curves (A), (B), (C) and (D) are 833.48 Oe, 833.4 Oe, 7.026 Oe and 5.7 Oe respectively.
The decrease in the coercivity of cobalt ferrite may attribute to magnetic interactions between the CoFe2O4 nanoparticles. From this figures we can observed only as particle size reduce, slight increases of the magnetization will be occur.
Magnetic hyperthermia temperature
To recognized temperature of cobalt ferrite (°C) as a function of MNP diameter (Table 1), we were measured using magnetic hyperthermia system. For the simplicity we indicate the four size distribution as the Average size (18, 25, 60 and 95 nm).
| Average size(nm) | Ambient T(°C) | T1 (°C) | T2 (°C) | T3(°C) | Mean T (°C) | ΔT (°C) | |
|---|---|---|---|---|---|---|---|
| A | 18 | 21.8 | 46.3 | 55.56 | 53.245 | 51.7 | 29.9 |
| B | 25 | 21.9 | 43.5 | 52.2 | 50.025 | 48.58 | 26.68 |
| C | 60 | 21.9 | 42 | 50.4 | 48.3 | 46.9 | 25 |
| D | 95 | 21.9 | 40.1 | 48.12 | 46.115 | 44.78 | 22.88 |
Table 1: Induction heat generating with different MNP size.
We can notes from Table 1 that the temperature (magnetic hyperthermia) changes with the different size of CoFe2O4, where the temperature decreases with increasing the particle size of CoFe2O4.
TEM measurement shows that size of the samples that were syntheses by co-precipitation method are in Nano size. The existence of stabilizer encapsulates atoms (oleic acid) 800 which prevent agglomeration and precipitation and thus get a smaller size. The size of particles increases with increase the preparation temperature due to more metal ions are produce which in turn increases the particle sizes, tiny amount of agglomeration is observed at higher temperature due to growing distribution of particle size. We noted that the size of CoFe2O4 nanoparticles was 10-25 nm, 15-35 nm, 20-100 nm and 75- 115 nm for the samples prepared at 600, 800, 900, and 1000 respectively. The measured magnetization curve for the sample that was syntheses by co-precipitation method display clearly ferrimagnetic behaviour and the magnetic property of CFNP depends on the particle size. The temperature increase (ΔT) of the tube content at 60 was 29.9°C for MNPs of 18 nm, 26.7°C for 25 nm, 25°C for 60 nm and 22.9°C for MNPs of 95 nm and the 18 nm exhibiting a high heating efficiency. The heating efficiency might be a combined effect of relaxation loss and hysteresis loss of the magnetic particles.