Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Research Article - (2014) Volume 4, Issue 3
Triggering drug release in tumor or disease sites at specific times can be one approach to treat diseases efficiently by limiting side effects from high systemic or off-target exposure. In this study we investigated triggered drug release of a liposome gel by magnetic heating from Iron Oxide Magnetic Nanoparticles (IMN). The liposome gel was prepared by self-assembly of drug encapsulated liposomes, IMN, and hydrophobically-modified chitosan (hmC) solution. The triggering release of the liposome gel was investigated in the Alternating Magnetic Field (AMF). In addition, AMF effect in cell toxicity of the doxorubicin liposome was evaluated. Drug release from the liposome gel via AMF demonstrated triggered release and enhanced cancer cell killing effect.
Keywords: Nanobiology; Drug release; Hydrogel; Nanoparticles; Drug delivery
Interest in controlled drug release for cancer or disease treatment has increased a lot recently [1-8]. Controlled drug release within a therapeutic dose window to a specific location and time can increase drug efficacy and minimize side effects at the same time by limiting exposure to the targeted tissue. To improve controlled drug release, many strategies have been devised to employ physical or chemical changes to trigger release of drug. Such ‘smart’ drug delivery systems have been designed to exploit temperature, enzyme, or pH conditions that are unique to the targeted tissue by constructing platforms from polymers, liposomes or inorganic nanoparticles [9-16]. The basic platform of controlled release is achieved by the development of a stimulus-sensitive drug carrier, often prepared by complex polymer or lipid synthesis methods. By combining the targeted molecules or implanting carriers these stimuli-sensitive drug carriers can be localized to the specific disease sites [17-19]. Controlled drug release can be triggered by drug carrier itself or by external stimuli (e.g., electrical or magnetic fields). In this study we limited our interests on triggered and controlled drug release by magnetic heating.
We used a hydrogel system containing Iron Oxide Magnetic Nanoparticle (IMN). Drug release from hydrogels has many advantages over free drug [20]. It prolongs drug release and prevents quick opsonization by direct blood circulation. Therefore, delivering drug using the hydrogel (especially injectable hydrogel) has gained more attention in recent years [21,22]. The assembled hydrogel differs from conventional thermosensitive hydrogel, which is usually using stimulus sensitive polymers, and uses the complex polymer synthesis. Our approach is to embed stimulus sensitive liposomes in a polymer gel matrix [23]. The liposome gel in this study is formulated by self-assembly upon mixing temperature sensitive Dipalmitoylphosphatidyl-Choline (DPPC) liposomes and hydrophobically modified chitosan (hmC). This assembly employs naturally temperature sensitive liposomes and a biopolymer scaffold, chitosan. Chitosan provides a diffusion barrier and liposome storage depot, and the stimulus sensitivity can be originated from the embedded liposome. The liposome gel system that can be injected and thereafter would release drugs for an extended period time [24]. In this case, it is important to maintain liposome stability in the gel matrix. The vesicle gels reported in the previous paper demonstrated the vesicle stability for several months and injectable properties for sustained local drug delivery [24]. In this study we investigated drug triggering by external stimuli, that is, whether IMN can trigger drug release more efficiently in a timely manner. Iron oxide nanoparticles have been proved that they can increase temperature by applying AMF [25]. Therefore, the incorporation of iron oxide nanoparticles into the liposome gel may trigger drug release more efficiently from the liposomes.
Materials
All chemicals were purchased from Aldrich-Sigma unless specified otherwise. All lipids (Dipalmitoylphosphatidyl-Choline (DPPC), 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N- [Methoxy(Polyethylene glycol)-2000] (DSPE-PEG2000)) were purchased from Avanti Lipids, Inc. (Alabaster, AL) and doxorubicin hydrochloric acid (Dox) was obtained from Bedford Laboratories (Bedford, OH). The chitosan and the hmC were identical to those used in the previous paper (molecular weight 200kDa, degree of deacetylation 80%) [26]. The graft density of n-dodecyl hydrophobes on the hmC was 2.5 mol%. Biogel A-0.5m was purchased from Bio-Rad Laboratories (Hercules, CA). IMN were either purchased (MicroMod GmBH, Rostock, Germany) or provided by Professor Hyeon’s research Group (Seoul National University, Korea). Easyheat heating system (2.4 kW, 182 kHz, Ameritherm Company, Scottsville, NY, USA) was used for magnetic heating.
Preparation of liposomes
A lipid film was formed by removing chloroform solvent under nitrogen at room temperature. SUVs (20 mg/ml) were prepared at 4 °C using a Probe Sonicator (W-375 Heat Systems-Ultrasonics, NY, USA) [24,27]. They were in the size range of 100 – 150 nm diameter, as determined by dynamic light scattering. Briefly, to encapsulate calcein as a model drug, a lipid film (20 mg/ml) was reconstituted in a self-quenched concentration of calcein (50 mM, pH 7.2, 1 mL). The liposomes were eluted through a size-exclusion gel chromatography column (Biogel A-0.5m, pre-equilibrated with pH 7.4 HEPES buffer) to remove free calcein. To load Dox into liposomes we used (NH4)2SO4 gradient exchange method [28-30]. Liposomes were prepared in the presence of ammonium sulfate (300 mM, pH 7.5). Eluted liposomes were collected and incubated with Dox at room temperature overnight. Dox-loaded liposomes were separated from free Dox using a size exclusion gel chromatography column (Biogel A-0.5m, Bio-Rad Laboratories, Hercules, CA) pre-equilibrated with pH 7.4 HEPES buffer. Calcein and Dox entrapment efficiency was determined by fluorescent intensity change at Ex/Em 495/515 nm and at Ex/Em 480/580 nm, respectively using a fluorescence microplate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA) after adding the detergent Triton X 100 (TX100, 0.02% final concentration) [24,27].
IMN and liposome characterization
A Malvern Zetasizer Nano ZS instrument (Southborough, MA) was used to measure the hydrodynamic diameter at 25°C. Three measurements were made per sample, and the averaged data was reported. Iron concentration was determined by UV-visible spectrophotometer as previously described [31].
IMN’s heating effect measurement
The heating effect of IMN can be evaluated by Specific Loss Power (SLP) value. The SLP value was determined by thermometer (IT-23, Physitemp, NJ, USA) by measuring temperature change before and after magnetic heating using Box-Lucas equation.
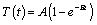 (1)
(1)
The temperature curve was fitted by two parameters, A and B and the SLP value was calculated based on following equation [32,33].
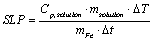 (2)
(2)
The coil temperature was controlled well by cooling air. In addition, the values were determined, measuring the temperature rise by an infrared themometer (FISO Technologies, Quebec City, Canada).
Preparation of the IMN-liposome gels
Liposome, IMN and hmC polymer mixtures of desired compositions were prepared by mixing the corresponding stock solutions as before [24,26]. Samples were vortexed and homogenized, followed by centrifugation to remove bubbles.
In vitro release studies
Phosphate-Buffered Saline (PBS) solution was used as the continuous medium. The drug concentration was monitored by fluorimeter (FluoroMax 3, HORIBA Jobin Yvon, Edison, NJ), UV-Vis spectroscopy and a fluorescent microplate (SpectraMax M2). To disrupt liposomes for determining the total drug concentration, the Triton X-100 detergent was used. In case of calcein release study, the mixture of prepared hmC solution (in 1% acetic acid solution) and IMN was added into a volume of free calcein solution or calcein containing liposome solution at room temperature. IMN- calcein liposome gels (DPPC 10 mg/mL, Calcein 25 mM, hmC 10 mg/mL) with calcein removed were prepared 200 μL of samples was placed into the bottom of UV/Vis plastic cuvette and PBS was added on the top of the gel. The supernatant of the vial was repeatedly monitored by the fluorimeter and the withdrawn solution was recovered to the vial after measurement.
Cell viability tests
Cytotoxicity by diffused drug through a membrane in a centrifugal filter tube (0.22 μm membrane, Fisher Scientific, UK) was evaluated with lymphoblastoid cells derived from a Burkitt lymphoma (Raji, ATCC CRL-2367). Raji cells were grown in DMEM media supplemented with 10% fetal serum, 2 mM L-glutamin, 100 U of penicillin/mL, and 100 μg of streptomycin/mL (GIBCO® media, Invitrogen, USA). The mixture of prepared hmC solution and IMN was added into a volume of free Dox solution or Dox containing liposome solution at room temperature. IMN-Dox liposome gel (DPPC 2 mg/mL, Dox 5 μg/mL, IMN 80 μg/mL, hmC 2 mg/mL) with free Dox removed formulation was placed into the bottom of a 2 mL centrifugal filter tube with 0.22 μm membrane and 2mL DMEM media was added to the gel. In the upper membrane chamber 1.3×106 Raji cells were loaded with DMEM media. After preparing the tube, it was placed in the middle of coil. The coil temperature was controlled well by cooling air. After applying AMF for 10 minutes to freshly prepared IMN-liposome gel, cells were taken from the upper membrane chamber and cell number was counted.
To see this short term hyperthermia effect of AMF we amplified the Dox release effect by incubating the Dox released solution from the tube into a 6 well cell culture plate for 2 days. Briefly, 1mL solution containing about 1 μg/mL Dox was withdrawn from the lower chamber after AMF applied and mixed with 4ml fresh DMEM media. The solution was transferred into 6 well plates loaded with 1.2×106 cells and cultured with the media in the plate. The cultures were maintained in a humidified incubator at 37°C with 5% CO2. Two days later cell viability was evaluated from trypan blue staining.
Data analysis
The average data and standard deviation of three samples were reported. Data are expressed as mean ± SD. Cell viability data are expressed as percentage of average values of the corresponding control Raji cells. Statistical analysis was performed using ANOVA, and the Student’s t test was performed for unpaired data between two groups. All tests were two-sided, and a probability value (P) of less than 0.05 was considered significant.
Triggered drug release from liposome gel by magnetic heating
We demonstrated drug release properties of our liposome gel in the previous paper [24]. In this study, we investigated triggered drug release using magnetic heating. The main triggering strategy is shown in (Figure 1). We prepared a liposome gel and mixed it with IMNs to induce magnetic hyperthermia. IMN is known to generate thermal energy through some combination of several potential loss mechanisms (e.g., Néel and Brownian relaxation) [25,34]. The generated heat depends on the physical properties of IMN (e.g. core size, core diameter, and shell type) and system parameters (e.g., frequency, coil dimension and turn number, and power). To evaluate the generated heat by different IMNs, the IMN’s SLP value was determined separately by measuring temperature increase before and after AMF application or continuous temperature reading by optic fiber temperature measurement. The result demonstrated that the temperature increased via magnetic heating and SLP value increased as the core size of IMN increased (supporting information). To induce hyperthermia effect in the liposome gel we selected an IMN that has sufficient loss power to generate useful heating (SLP=560.3W/g, 121 Oe, core size 20nm, BNF IMN (MicroMod, size 80 nm). As the IMN loading amount increased, the prepared liposome gel becomes darker (Figure 2). The swelling index (defined by swelled gel height in the PBS / initial height of the IMN-liposome gel (i.e., before adding the PBS solution) was 2.3, and this value was nearly identical with all the samples. This indicated that liposome gel properties were not affected by the presence of the IMN. A clear boundary between the PBS solution and the gel was shown. It indicates that calcein could release into the PBS buffer solution while still maintaining the integrity of a liposome gel. With different IMN amounts loaded liposome gels we studied AMF-dependent drug release. Fluorescence intensity was measured before and after applying Alternating Magnetic Field (Figure 3). The fluorescent signal increased abruptly after exposing the IMN liposome gel into AMF and the more IMN concentration showed more quick release response. As a result, AMF demonstrated that triggered drug release could be feasible from the IMN-liposome gel. It suggests that AMF initiates more quick release than the control release (i.e., without AMF application). We also studied AMF dependent repeated drug release experiment by turning AMF machine on and off repeatedly. As shown in (Figure 4), drug release strongly depends on AMF field within this time period and the signal increase was obvious. Of note, control experiment of a liposome gel without IMN did not show fluorescent signal increase clearly. Drug release from the liposome gel was time dependent and AMF study demonstrated that it triggers drug release more quickly.
Figure 1: Structure of an Iron Oxide Magnetic Particle (IMN)-doxorubicin (Dox) liposome gel (left) and under magnetic heating (right). The hydrophobically modified chitosan (hmC) inserts its hydrophobes into liposomal bilayers and thereby connects the liposomes into a gel while IMNs disperse in the gel, as shown on the left. When Alternating Magnetic Field (AMF) is applied, the liposome becomes leaky because of the lipid bilayer melting and doxorubicin is released.
Figure 2: A photograph of an IMN-liposome gel that is composed of calcein encapsulated DPPC/hmC liposome gel (DPPC 10mg/mL, Calcein 25mM, hmC 10mg/mL) and IMNs (Fe amount 0, 20, 50, 100, 200, 400 μg/mL from left to right),demonstrating calcein release into the PBS solution and a clear boundary between the PBS solution and the gel below that still maintains the integrity of a liposome gel in the PBS. The color of IMN-liposome gel becomes darker as the IMN concentration increases.
Figure 3: AMF effects on calcein release profile from the IMN-liposome gel composed of calcein encapsulated liposome gel (DPPC 10mg/mL, Calcein 25mM, hmC 10mg/mL) C) + IMN. Fluorescent signal increased abruptly after exposing the IMN- liposome gel into AMF and the increased amounts of IMN gave faster response and great change in the fluorescent signal.
Figure 4: Repeated calcein release from IMN-liposome gel composed for calcein encapsulated liposome gel (DPPC 10mg/mL, Calcein 25mM, hmC 10mg/mL) + IMN (400 μg/mL) by turning AMF machine on and off repeatedly, demonstrating drug release strongly depends on AMF field within this time period and above a certain point the signal increase was obvious, presumably due to temperature increase.
Cell killing effect of amf triggered liposome gel
To evaluate cell killing effect of the liposome gel by AMF, Dox encapsulated DPPC liposome gel was prepared and mixed with BNF IMN. The IMN-liposome gel (DPPC 2 mg/mL, Dox 5 μg/mL, IMN 80 μg/mL, hmC 2 mg/mL) demonstrated a clear boundary between the DMEM media and the gel. After AMF application Dox was released out and the color was changed into red (Figure 5). Cells were taken from the upper membrane chamber and cell number was counted. It did not show any difference between AMF applied sample and control sample (no AMF applied sample). The result showed that cell killing by the diffused Dox was not achieved quickly and the temperature in the tube did not increase in the media, rather maintained constant because the magnetic hyperthermia effect was restricted to the local heating. Therefore, to see this short term hyperthermia effect of AMF the Dox release effect was amplified by incubating the Dox released solution from the tube into a 6 well cell culture plate for 2 days. The result demonstrated that AMF applied sample had more toxic effect than non-AMF applied sample because the liposome gel exposed by AMF even for 10 min can lead a slightly increased drug release p<0.01, n=3, (Figure 6). Dox release from non-AMF applied sample was caused by Dox diffusion through a chitosan gel matrix and from drug encapsulated liposomes. Dox release from AMF applied sample was enhanced by magnetic heating. Of note, IMN embedded liposome gel demonstrated a strong gel property even in the DMEM media, which was also observed when the liposome gel was prepared in the DMEM media, the gel was washed with the media three times by inverting the tube and it maintained the gel strength (Figure 7). In contrast, Dox+hmC mixture and hmC solution could not maintain the gel integrity in the DMEM media and lost the most of the contents by the washing process. Interestingly, the liposome gel to which AMF was not applied maintained the gel strength at least 2 weeks in the DMEM media but the liposome gel applied with AMF field lost the gel strength 4-5 days later.
Figure 5: Schematic and photographs of the experimental setup: a) schematic drawing; b) IMN-Dox liposome gel before AMF; c) IMN-Dox liposome gel after AMF. An IMN-Dox liposome gel (DPPC 2mg/mL, Dox 5μg/mL, IMN 80 μg/mL, hmC 2mg/mL) was added to the bottom of the lower tube. In the upper membrane chamber 1.3x106 Raji cells were loaded. Before AMF, a photo clearly demonstrated the upper chamber that has a pink culture media color and the lower chamber that has an IMN-liposome gel and a brown color buffer solution. After AMF, Dox was released out and the color has been changed into red
Figure 7: Photographs of gel strength measurement by inverting tube: a) IMN-liposome gel composed of Dox-PPC+hmC+ IMN; b) the mixture of Dox+IMN+hmC; and c) the mixture of Dox + hmC. The samples were kept in the cell culture media for 3days and the tubes were inverted each day. The IMN-Dox liposome gel demonstrated that it can remain gel state in the culture media.
Triggered Drug release from the liposome gel via AMF can be initiated by several paths. Applied AMF can heat up IMN through some combination of several potential loss mechanisms (e.g., Néel and Brownian relaxation), depending upon nanoparticle properties and experimental conditions. This AMF induced heating can heat up liposomes locally, cause the phase transition of liposome, and release drugs from the inside of liposome [35]. To make this scenario feasible, we need 1) IMNs that can be heated up efficiently by AMF, 2) the amounts of IMN that are enough to heat up liposome bilayers to induce a phase transition, 3) liposomes that can remain intact in the system without drug leakage before AMF application and can be triggered specifically by heat. Previously we demonstrated different vesicles can be formulated in the vesicle gel. The results were promising to conduct the current approach. With this information, we first investigated whether it can accommodate temperature dependent liposome formulations and still keep temperature dependent liposome property in the gel (Supporting Information). The results showed that the liposome gel successfully achieved this goal. Triggered drug release from the liposome gel was investigated by calcein and cationic therapeutic drug (Dox) release at different temperatures. Calcein and Dox release from the DPPC liposome gel indicates a temperature dependent release. The temperature dependence of Dox and calcein release from DPPC liposome solution was also reported and confirmed [36, 37]. It suggests that the liposome gel formulation and the preparation process do not interfere significantly with temperature sensitive liposomes and reflects that the preparation is a relatively quick process that makes liposome intact in the gel, presumably by minimizing liposome destabilization and reorganization. Depending on the liposome formulation the drug release amount from the liposome gel matrix can be different. The maximum drug release rate at different temperature can be correlated with lipid melting temperature. For example, the drug release rate from DPPC liposomes increases at its lipid melting temperature (i.e., 41°C). L-α-phosphatidylcholine (e.g., Egg PC) liposome has lower melting temperature (i.e., -10°C). Therefore, the drug release rate of Egg PC formulations above body temperature does not increase from the room temperature. In contrast, the drug release rate of DPPC formulations increases in the body temperature. Egg PC liposome gel did not show any temperature dependence above room temperature.
As next steps, IMN that can be used for this temperature dependent drug release from the IMN-liposome gel have been screened and based on SLP value, the amount of IMN was decided to heat up the liposome gel system. Interestingly, the IMN amount did not change liposome gel property (Figure 2). The selected IMN demonstrated magnetic heating effects and triggered release pattern, which indicates that the SLP value and the IMN amount are in the right range to trigger drug release in the liposome gel.
For cell killing applications via AMF, the liposome gel may need instant drug release by AMF application. Therefore, it was expected that when AMF is applied to the tube, Dox is released out from the IMN-liposome gel, Dox concentration increases in the tube, and finally Dox diffuses to the upper chamber and kills the cells in the upper chamber. Another effect by AMF will be a temperature increase in the tube. If the temperature increases more than 42°C, cells are killed by the hyperthermia effect. However, cell killing effects by AMF were not easily evaluated and detected quickly. Several factors should be considered: time lapse that exists to evaluate cell killing effect because cell death and growth require time; initial loaded drug amounts that determine cell killing; and IMN amounts that determine heating. In the tube test experiment with the IMN-liposome gel in a tube, initial drug release by AMF in the DMEM media for less than 30 min was not sufficient enough to kill cells instantly as discussed in other papers [38]. However, the cell killing experiment that we performed by amplifying Dox release suggests that small amounts of drug leakage by AMF are enough to kill cells eventually. Of note, the gel strength lost after AMF application. It suggests that losing gel strength and liberating liposomes quickly by AMF can occur to accelerate drug release.
The previous study demonstrated that the liposome gel could release drug for an extended time from two depots – from a chitosan gel matrix and from drug encapsulated liposomes. The injected or implanted vesicle gel can provide drug encapsulated liposome depot in the body, as a result. The application of AMF can control drug release time by changing gel property and bursting drug encapsulated liposomes because AMF applied liposome gel can speed up liposome liberation and make liposome leaky. The reason should be further investigated. Presumably, the applied AMF can lead iron oxide nanoparticles to heat up in the gel, give heat locally to the lipid bilayer, destruct interconnected hm-chitosan (hmC) linking, and may change the gel strength eventually. Thus, it suggests that IMN-liposome gel can be used as a prolonged drug release platform for long term drug release and a controlled release depot delivered drugs at desired times.
In this work, we have studied an AMF-triggered drug release from IMN embedded liposome gel, a mixture of an associating biopolymer, hm-chitosan (hmC) and drug encapsulated liposomes. We have shown that this liposome gel can be formulated with Iron Oxide Magnetic Nanoparticles (IMN) to facilitate magnetic heating effects of IMN. Currently, the usage of magnetic heating by IMNs can be limited due to the relatively high amounts of IMN to induce direct heating to a specific location. Interestingly, in this study we demonstrated that with small amounts of IMN drug-encapsulated liposomes can trigger drug release in the liposome gel and control drug release, which uses a very mild condition, not inducing a bulk gel heating. The results from this study indicate that the liposome gel system can be used for the triggered and sustained drug delivery of anti-cancer drugs for implantable applications.
This work was supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research. We thank Dr. Blumenthal’s group members (especially, Dr. Anu Puri) for discussions and help. We also thank Dr. Taeghwan Hyeon’s research group at Seoul National University (especially Dr. Byung-Hyo Kim and Jisoo Lee) for providing iron oxide nanoparticles.