Pediatrics & Therapeutics
Open Access
ISSN: 2161-0665
ISSN: 2161-0665
Research Article - (2023)Volume 13, Issue 4
Prompt and accurate diagnosis and parasite density quantification are keys to effective malaria treatment. The malaria diagnostic and parasite density quantification methods currently in use are not without their shortcomings. Thus, there is the need for new methods with high sensitivity, specificity and predictive values that may complement other methods in use. In this study, 100 children (1 years-10 years) diagnosed of severe malaria were treated with 2.4 mg/kg body weight of artesunate intravenously at 0 hour and then 1.2 mg/kg body weight at 12 hours, 24 hours and 48 hours, followed by artemether-lumefantrine combination therapy at doses of 4 mg/kg body weight of artemether and 24 mg/kg body weight of lumefantrine for three days, with 200 clinically healthy children of the same age range serving as control. The diagnostic potential of selected biochemical parameters in the serum of patients before treatment (day 0), 48 h of treatment (day 2) and 48 h after treatment (day 7) were evaluated. Using regression analysis, different relationships between the identified predictors and the predicted variable (parasite density) were tested. Serum Glutathione Reductase (GR) activity at >85.78 U/l (cut-off value) was indicative of severe malaria and had the highest diagnostic values with 98.5% sensitivity, 96% specificity, 98.01% positive predictive value, 4.04% negative predictive value and 1576 as odds ratio, with an area under curve of 0.999, comparing favourably with microscopy (gold standard) at 95% confidence interval. This was followed by elevated serum glutathione peroxidase activity. Other parameters had relatively high sensitivity with relatively low specificity or vice versa. The polynomial quadratic equation, parasite density (×103 parasites/μl)=6.96 (GR)-433.1-0.01 (GR)2 had the best good-fit for prediction of parasite density, using serum GR activity. Thus, serum GR activity may be a very effective diagnostic and parasite density prediction tool for severe Falciparum malaria in children.
Diagnosis; Falciparum malaria; Parasite density glutathione reductase; Artesunate/artemether-lumefantrine combination therapy; Children
The high prevalence of malaria morbidity and mortality among children especially in endemic countries coupled with the emergence of drug resistant species to conventional antimalarial drugs prompted the World Health Organization (WHO) in 2006, to shift from presumptive clinical diagnosis to confirmatory laboratory diagnosis of malaria, with artemisinin combination therapy recommended for the treatment of malaria [1]. This is to delay the emergence of resistance to artemisinin drugs. Consequently, accurate, precise and timely diagnosis of malaria and parasite quantification has become a matter of great concern [2].
The various methods of laboratory diagnosis of malaria include microscopy using Giemsa stained blood smears, being the WHO certified gold standard for both diagnosis and parasite quantification, quantitative buffy coat immunochromatic methods, serology tests and polymerase chain reaction methods. These methods currently in use are not without their shortcomings [3].
Though, diagnosis and parasite quantification using microscopy is less expensive and a valuable technique when performed correctly by an expert, it is affected by inter reader variability and requires technical expertise. Immunochromatic methods, which are Rapid Diagnostic Tests (RDTs) which do not require laboratory support, are far more costly than conventional microscopy. They do not permit quantification of the parasite and various RDTs cross react with autoantibodies such as rheumatoid factors, resulting in false positive results and less sensitivity. Though, nested and real time polymerase chain reaction present higher sensitivity and specificity to malaria diagnosis and quantification compared to light microscopy, they suffer the frail of easy contamination and mutation, require expertise and are very expensive, making them not easily accessible in developing countries where malaria is endemic and accurate diagnosis most needed. Due to the shortcomings of the conventional methods and the need to improve on diagnosis of falciparum malaria and parasite quantification, especially in children, there is the need to explore new diagnostic and quantification approaches to malaria [4].
The diagnostic ability of a parameter or test method for a disease is defined by its diagnostic values which take sensitivity and specificity into account for accuracy. Sensitivity is the probability of the parameter giving a positive result when the patient actually has malaria while specificity is the probability of the parameter giving a negative result when the patient actually does not have malaria. A reliable disease diagnostic parameter or tool should have sensitivity and specificity greater than 90% [5].
Microscopy using the parasites/μl method by comparing the ratio of counted parasites within a given number of microscopic fields against either counted White Blood Cells (WBCs) or counted Red Blood Cells (RBCs) within those same fields, and then multiplying that ratio by either the measured or estimated density of WBCs or RBCs in the blood of the patient is the conventional method of parasite quantification. Due to the frequent unavailability of equipment and facilities in some malaria endemic countries to quantify WBCs, an assumed WBCs count of 8000/μl of blood was accepted by world health organization as reasonably accurate to estimate malaria parasite densities.
The diagnostic values of some haematological parameters were reported by Poluga, et al., and Maina et al. in uncomplicated malaria. Eze, et al., derived mathematical models for prediction of parasite density using haematological parameters in adult male subjects. The use of mathematical models for predicting the density of P. falciparum using biochemical parameters with high sensitivity, specificity and precision for malaria diagnosis has not been elucidated. This, if elucidated could provide an opportunity to improve on quantification of Plasmodium falciparum density in children, thereby helping to reduce malaria morbidity and mortality [6].
We previously, reported on the responses of selected haematological and biochemical parameters to artesunate/ arthemether-lumefantrine combination therapy in children with severe malaria; however, the diagnostic and parasite quantification potentials of these parameters remain to be explored. Therefore, this study was carried out to evaluate the diagnostic and parasite quantifying potentials of selected biochemical parameters in the serum of severe malaria patients.
Study population
This study was carried out from 28th April 2014 to 15th February, 2016 at the paediatric wards of Jos University Teaching Hospital (JUTH), Jos, Plateau state, Nigeria. The study population consist of 100 children with severe malaria (case children) aged 1 to 10 years treated at the children’s wards of JUTH which is a tertiary hospital, serving many states in the country especially in the North-central region. The control children consist of 200 clinically healthy children (1 years to 10 years) without malaria attending the hospital for medical checkups and routine immunization [7].
Children who met the inclusion criteria of the study were recruited consecutively for the study. The inclusion criteria for the case children were:
• Children aged 1 years to 10 years clinically presenting severe
malaria without any other ailment as diagnosed by the
paediatrician (severe malaria was diagnosed as the presence of
one or more symptoms of malaria complications e.g. anaemia,
respiratory distress, jaundice, etc, and detection of malaria
parasite/product in the patient’s blood).
• Assent of the child and parent/care-giver’s consent.
• Children microscopically confirmed to have hyperparasitaemia.
• Children confirmed by laboratory tests as presenting only
severe malaria after excluding other disease conditions such as
septicaemia, sickle cell disease, typhoid, Glucose-6-Phosphate
Dehydrogenase Deficiency (G6PDD), Hepatitis B; shigellosis,
helminthiasis and Human Immune-deficiency Virus (HIV).
• Children on artesunate/artemether-lumefantrine combination
therapy.
• Children on admission in the hospital using mosquito
bed-net.
• Children that were discharged by the 7 days of admission
after recovery.
The control children were recruited based on the following inclusion criteria:
• Assent of the child and parent/caregiver.
• Children aged 1 years-10 years diagnosed as clinically healthy
by the paediatrician.
• Children confirmed by laboratory tests as clinically healthy.
• All children enrolled as control were negative for malaria
parasite using thick-smear examination.
• They were not on antimalarial drugs for the past 2 weeks or
on acetaminophen in the past 24 h, without febrile episodes in the past 6 months and without any sign of anaemia or
neurological involvement.
Study design
The study was a prospective hospital based case control study with diagnostic design.
Administration of drugs
Case children were intravenously administered with 2.4 mg/kg body weight of artesunate at 0 hours, and then 1.2 mg/kg body weight was given orally to them at 12 hours, 24 hours and 48 hours. This was followed by oral administration of artemetherlumefantrine combination therapy at doses of 4 mg/kg body weight of artemether and 24 mg/kg body weight of lumefantrine for three days [8].
Sample collection and biochemical analyses
Five millilitres (5 ml) of blood was aseptically collected from the ante cubital vein of both case and control children using sterile needle and syringe after clinical assessment. In the control children, this was done once while in the case children, this was done three times i.e. before the start of treatment on the day of admission (day 0), then 48 hour after commencement of treatment (day 2). Another sample was collected 48 hours after the last dose of the combination therapy i.e. 7th day of initiation of treatment [9].
Two millilitres (2 ml) of the blood was dispensed into EDTA tube for screening tests for exclusion of other abnormalities, malaria parasite and haematological tests. The screening tests carried out include: Blood culture this was used to rule out septicaemia by direct aseptic injection of the blood into brain heart infusion broth and thioglycollate broth (at 1:20 dilution) as was described by Cheesbrough; Glucose-6-Phosphate Dehydrogenase (G-6-PD test was used to exclude G6PD deficiency using the meth-haemoglobin qualitative method described by Brewer, et al. haemoglobin genotyping for exclusion of sickle cell disease using electrophoresis as described by Roberts and Williams; Hepatitis B surface antigen test was used to exclude viral hepatitis using Rapid Diagnostic Test (RDT) kit from standard diagnostics, Korea [10].
The remaining 3 ml of the blood was dispensed into screw caped plain sample tube. It was allowed to clot and retract at room temperature (22℃-27℃) for about 20 min. The serum was separated after centrifuging at 3000 rpm for 5 minutes in a clinical bench top centrifuge (MSE minor, England) using Pasteur pipette into pre-cleaned, dried, metal and steroid free cryo-vials for analysis of the biochemical parameters and HIV screening using the immunochromatographic technique (RDT; Standard Diagnostics, Korea).
Transparent stool containers were used to collect stool sample once from both case and control children before enrolment. This was used for exclusion of helminthiasis and pathological enteric bacterial infection, using stool microscopy and culture tests. Normal saline method was used for microscopy while selenite-F and dextrose-citrate agar were used for culture as described by Cheesbrough.
Malaria diagnosis: Duplicate thick and thin blood smears/films of Giemsa stained slides were used for microscopic examination for malaria parasite. Serving as the internal quality control are the duplicate slides read blindly by an expert in microscopy (a medical/clinical laboratory scientist) who was involved in the study. The slides were counter read by another expert in microscopy who was not involved in the study (independent reader) this served as the external quality control. Malaria diagnosis was based on identification of asexual stages of plasmodium species in the thick blood smears. Smear was reported as negative after examining about 100 fields. Thin smears were used to identify species and stages of plasmodium species. Malaria parasite density was determined by the number of parasites/μl of blood (thick smear using the total white blood cell count of respective patients. Hyperparasitaemia in children was defined as parasite count >200 × 103 parasites/μl.
Parasite density (parasite/μl of blood)=(N × total WBC counts)/ (leucocytes count (200))
Where;
N=number of asexual forms of the parasite counted in 200 leucocytes count.
Determination of biochemical parameters in serum: Glutathione Reductase (GR) activity was determined by monitoring the reduction of oxidized glutathione to the reduced form in the presence of β-Nicotinamide Adenine Dinucleotide Phosphate (NADPH), which is oxidized to NADP+, following the procedures of Goldberg and Spooner. Glutathione Peroxidase (GPx) activity was determined by measuring the rate of oxidation of glutathione according to the method of paglia and valentine. Superoxide Dismutase (SOD) activity was determined by measuring the level of inhibition of epinephrine according to the method of Hara and Irwin. The serum level of glucose was determined using glucose oxidase method as described by Barham and Trinder. Bicarbonate concentration was quantified spectrophotometrically by phosphoenol pyruvate carboxylase method as described by Tietz. Phosphoenol Pyruvate Carboxylase (PEPC) catalyses the reaction between phosphoenol pyruvate and carbondioxide (bicarbonate) to form oxaloacetate and phosphate ion. Oxaloacetate is reduced to malate with simultaneous oxidation of an equimolar amount of reduced Nicotinamide Adenine Dinucleotide (NADH) to NAD; the reaction is catalysed by Malate Dehydrogenase (MDH). This results in a decrease in absorbance at 340 nm that is directly proportional to CO2 concentration in the sample. Protein Carbonyls (PCO) concentration was determined following the method described by and Reznick and Packer whereby 2, 4- dinitrophenylhydrazine reacts with protein carbonyls, forming a Schiff base to produce the corresponding hydrazone, which was analysed spectrophotometrically [11].
Quality control: The sample, control and standard solutions were analysed for biochemical parameters (GR, GPx, SOD, glucose, bicarbonate and PCO) using duplicate tubes. They were assayed spectrophotometrically using chemistry auto-analyser, model; Mispa Excel version 2.1e lite (open system) 2006 by Mispa Biosystems India. The assays were performed by certified medical/clinical laboratory scientists.
Determination of diagnostic indices of selected serum biochemical parameters: A 2 × 2 contingency table was used to determine the number of True Positives (TP), False Positives (FP), True Negatives (TN) and False Negatives (FN) for the potential new method (s) against the gold standard (microscopy). The sensitivity, specificity, predictive values and odds ratios of the potential new diagnostic methods were then calculated using manual statistical methods. Receiver Operational Characteristic (ROC) curve was prepared by plotting the sensitivity (true positive) against 1-specificity (false positive) of the serum biochemical parameters. This was used to further confirm the cut-off values and sensitivity of the parameters in malaria diagnosis.
Statistical analysis
Data are presented as means ± S.E.M and means ± Standard Deviation (SD). One-way Analysis Of Variance (ANOVA), post hoc Duncan multiple range test and regression analysis were used for the analysis of the data using Statistical Software for Social Sciences (SPSS) (version 19.0, SPSS Inc., Chicago, IL). Differences between means at 95% confidence interval were considered significant.
According to Okoli, et al., the responses of the parameters were categorized as: Fast response (those that increased or decreased steadily and their means by day 7 were not significantly different from the controls); fastest response (those that their means increased or decreased such that there was no significant difference between their levels on days 2 and 7 compared to controls); and slow response (those that increased or decreased steadily but have means which were significantly different on day 7 compared to controls) [12].
% discrepancy=((Parasite density by microscopy-parasite density by GR method)/(parasite density by microscopy+parasite density by GR method)) × 100
From our previous report, the results revealed that one hundred percent of the children presented three or more signs of severe malaria before treatment which resolved progressively with treatment. Fever, anaemia, jaundice, respiratory distress and prostration were the most common signs before treatment. One hundred percent of the children recovered and were discharged by the 7th day of admission.
Malaria diagnostic potentials of the parameters
Serum biochemical parameters in children with severe Falciparum malaria: There was a steady significant (p<0.05) rise in serum GR and GPX activities, PCO concentration, PCO/SOD ratio with rise in parasite density before treatment compared to controls, which were decreased significantly (p<0.05) with treatment compared to values obtained before treatment. Serum SOD activity decreased significantly (p<0.05) with rise in parasite density compared to control and increased significantly (p<0.05) with treatment compared to values obtained before treatment (Table 1). There was no significant alteration (p>0.05) in serum glucose concentration with increasing parasite density from 201 to 600 × 103 parasites/μl, though it was significantly lower (p<0.05) at parasite density between 601 to 800 × 103 parasites/μl compared to lower parasite densities before treatment (day 0). There was no significant difference (p>0.05) in the glucose level after treatment (day 7) compared to the level before treatment (day 0) at parasite density between 601 to 800 × 103 parasites/μl (Table 1). There was no significant difference (p>0.05) in the levels of HCO3- before, during and after treatment (Table 1).
| Range of parasite density | GR activity (U/l) | GPx activity (U/l) | SOD activity (U/ml) | PCO conc. (nmol/ml) | Glucose conc. (mmol/l) | HCO3-conc. (mmol/l) | PCO/SOD ratio | HCO3-/glucose ratio |
|---|---|---|---|---|---|---|---|---|
| Day 0 (before treatment) | ||||||||
| Mild hyperparasitaemia | ||||||||
| 201-300 (× 103 parasites/µl) | 153.71 ± 12.01c | 183.66 ± 27.77bc | 1.26 ± 0.16a | 168.10± 7.10c | 4.83 ± 0.21c | 21.91 ±.54ab | 305.15 ± 52.26a | 4.31 ± 0.32ab |
| Moderate hyperparasitaemia | ||||||||
| 301-400 (× 103 parasites/µl) | 150.40 ± 4. 56c | 251.89 ± 23.88cd | 1.35 ± 0.23a | 177.06 ± 10.78c | 4.79 ± 0.27c | 22.69 ± 0.94bcd | 262.13 ± 59.26a | 4.44 ± 0.39abc |
| 401-500 (× 103 parasites/µl) | 174.37 ± 8.30c | 684.55± 130.83f | 0.49 ± 0.09a | 245.03± 2.62d | 4.28 ± 0.39c | 23.52 ± 1.08bcde | 3029.12 ± 109.40b | 5.82 ±0.44bcd |
| Marked hyperparasitaemia | ||||||||
| 501-600 (× 103 parasites/µl) | 208.24 ± 2.58d | 359.19 ± 5.33de | 0.21 ± 0.07a | 274.44 ± 5.47e | 4.20 ± 0.41c | 17.39 ± 1.55a | 2044.64±276.60b | 4.07 ± 0.44ab |
| 601-700 (× 103 parasites/µl) | 225.32 ± 5.15d | 455.19± 44.93e | 0.03 ± 0.001a | 280.20 ± 0.04e | 3.49 ± 0.02a | 27.83 ± 0.1e | 10377.50 ± 1.50c | 7.0 ± 0.1d |
| 701-800 (× 103 parasites/µl) | 254.70 ± 3.80e | 643.93±37.45f | 0.085 ± 0.06a | 298.54 ± 12.54e | 3.67 ± 0.10a | 24.58 ± 1.59cde | 9607.06 ± 648.31c | 5.83 ± 0.45bcd |
| Day 2 (48 hours of treatment) | ||||||||
| MP not seen (treated MP negative | ||||||||
| 0.0 (× 103 parasites/µl) | 64.28 ± 1.68a | 107.37± 3.48abc | 0.67 ± 0.01a | 84.23 ± 1.92b | 4.77 ± 0.02b | 23.00 ± 0.2bcd | 124.86 ± 2.82a | 4.0 ± 0.01ab |
| Low parasitaemia | ||||||||
| 01-100 (× 103 parasites/µl) | 95.35 ± 1.44b | 139.20±1.92abc | 1.23 ± 0.19a | 107.93 ± 2.40b | 5.03 ± 0.19c | 19.29 ± 2.48ade | 205.06 ± 45.10 a | 3.11 ± 0.21a |
| Moderate parasitaemia | ||||||||
| 101-200 (× 103 parasites/µl) | 113.40 ± 0.94b | 175.78± 1.81bc | 1.03 ± 0.09a | 154.52 ± 2.71c | 4.17 ± 0.13a | 26.73 ± 0.42de | 279.65 ± 23.68a | 6.35 ± 0.20cd |
| Day 7 (48 hours after treatment) | ||||||||
| MP not seen (treated MP negative | ||||||||
| 0.0 (× 103 parasites/µl) | 66.31 ± 0.93a | 28.26 ± 0.54a | 2.87 ± 0.20b | 47.74 ± 1.98a | 4.16 ± 0.10a | 24.44 ± 0.40cde | 27.28 ± 2.90a | 5.84 ± 0.24bcd |
| Low parasitaemia | ||||||||
| 01-100 (x 103 parasites/µl) | 93. 06 ± 2.92b | 50.79 ± 9.94ab | 4.88 ± 0.43c | 83.22 ± 13.83b | 4.05 ± 0. 34a | 20.39 ± 2.02ab | 19.14 ± 4.90a | 9.14 ± 0.40e |
Note: Each value is a means ± S.E.M. of 100 replicates. Values carrying different superscripts in the same column are significantly different (p<0.05).
Table 1: Selected biochemical parameters in serum of children with severe falciparum malaria.
Normal range of biochemical parameters in children and cut-off values for definition of malaria positives.
The normal serum ranges for GR, GPx, SOD, PCO, HCO3, glucose, PCO/SOD, HCO3/glucose in clinically certified healthy children (control) were determined. For the parameters (GR, GPx, PCO, glucose and PCO/SOD ratios), which had their values elevated due to malaria before treatment, the cut-off values for malaria positive were defined as values greater than the normal ranges (means plus 2 standard deviations of the control values) and those within the normal range were termed malaria negative. For the parameters (SOD, HCO3 and HCO3/ glucose) which had their values reduced due to malaria before treatment, the cut-off values for malaria positive was defined as values lower than the normal ranges (mean minus 2 standard deviations of the control values) while those within the normal ranges were termed malaria negative (Table 2) [13].
| Parameters | Children with severe malaria (day 0) Mean ± S.D (n=100) |
Control Mean ± SD (n=200) | Normal range Control Mean ± 2SD | Cut-off values for malaria positive |
|---|---|---|---|---|
| Glutathione reductase activity (U/l) | 183.94 ± 58.11 | 63.04 ± 11. 37 | 40.30-85.78 | >85.78 |
| Glutathione peroxidase activity (U/l) | 392.62 ± 44.30 | 19.49 ± 6.51 | 6.47-32. 51 | >32.51 |
| SOD activity (U/ml) | 0.76 ± 0.26 | 2.88 ± 1.025 | 0.83-4.93 | <0.83 |
| PCO (nmol/ml) | 223.22 ± 65.46 | 35.24 ± 13.62 | 21.62-48.86 | >48.86 |
| HCO3 ion concentration (mmol/l) | 22.28 ± 5.10 | 24. 55 ± 3.54 | 17.47-31.63 | <17.47 |
| Glucose concentration (mmol/l) | 4.40 ± 1.28 | 3.85 ± 0.71 | 2.43-5.27 | >5.27 |
| PCO/SOD | 2880.66 ± 463.04 | 65.60 ± 18.45 | 28.69-102.50 | >102. 50 |
| HCO3/Glucose | 4.85 ± 2.04 | 6.05 ± 1.63 | 2.78-9.32 | <9.32 |
Note: HCO3: Biocarbonate; PCO: Protein Carbonyl; SOD: Superoxide Dismutase; Day 0=before treatment.
Table 2: Cut-off values of some serum biochemical parameters for diagnosis of falciparum malaria in children.
Malaria diagnostic potential of selected serum biochemical parameters in children with severe falciparum malaria treated with artesunate/artemether-lumefantrine combination therapy.
Serum GR activity, above the cut-off value of >85.78 U/l, indicative of malaria, had the best diagnostic values (98.5% sensitivity, 96% specificity, 98.01% Positive Predictive Value (PPV), 4.04% Negative Predictive Value (NPV) and 1576 Odds Ratio (OR)) for diagnosis of malaria in children compared to other parameters. This was followed by serum GPx activity with cut-off value of >32.59 U/l (indicative of malaria), and sensitivity of 98%, specificity of 88%, NPV of 13.04%, PPV of 94.23% and an OR of 359.37 at 95% confidence limit. The other parameters had relatively high sensitivity with relatively low specificity or vice versa, indicating that they are not good malaria diagnostic indices (Table 3) [14].
| Biochemical parameters | TN (N) | FP (N) | Total (N) | TP (N) | FN (N) | Total (N) | Sen. (%) | Spec. (%) | NPV (%) | PPV (%) | OR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GR (>85.78U/l) | 96 | 4 | 100 | 197 | 3 | 200 | 98.5 | 96 | 4.04 | 98.01 | 1576 |
| GPx (>32.51 U/l) | 88 | 12 | 100 | 196 | 4 | 200 | 98 | 88 | 13.04 | 94.23 | 359.37 |
| SOD(<0.83 U/ml) | 88 | 12 | 100 | 114 | 86 | 200 | 57 | 88 | 6.9 | 90.48 | 1.79 |
| PCO (>48.86 nmol/ml) | 65 | 35 | 100 | 200 | 0 | 200 | 100 | 65 | 53.85 | 85.11 | 5.71 |
| Glucose (˃5.27 mmol/l) | 83 | 17 | 100 | 40 | 160 | 200 | 20 | 83 | 7 | 70.18 | 1.19 |
| HCO3 (<17.47 mmol/l) | 96 | 4 | 100 | 29 | 171 | 200 | 14.5 | 96 | 1.5 | 87.88 | 4.07 |
| PCO/SOD (˃102.49 nmol/U) | 88 | 12 | 100 | 142 | 58 | 200 | 71 | 88 | 8.21 | 92.2 | 18.83 |
| HCO3/glucose (<9.32) | 96 | 4 | 100 | 17 | 183 | 200 | 8.5 | 96 | 1.43 | 80.95 | 2.23 |
Note: GR: Glutathione Reductase; GPx: Glutathione Peroxidase; SOD: Superoxide Dismutase; HCO3: Bicarbonate, PCO: Protein Carbonyl; CRP: C-Reactive Proteins; TN: True Negative; FP: False Positive; TP: True Positive; FN: False Negative; sen: sensitivity; spec: specificity; NPV: Negative Predictive Value; PPV: Positive Predictive Value; OR: Odds Ratio
Table 3: Diagnostic indices of selected serum biochemical parameters in children with severe falciparum malaria treated with artesunate/artemether-lumefantrine combination therapy.
Figure 1 shows further confirmation of the diagnostic values of the selected parameters using the Receiver Operational Characteristic (ROC) curve. Serum GR and GPx activities, PCO, PCO/SOD had their curves above the mid-diagonal reference line and tended towards left, indicating high sensitivity in malaria diagnosis while serum SOD activity, HCO3 ion, glucose and HCO3/glucose ratio had curves below the line and tended towards right, indicating low accuracy in malaria diagnosis (Figure 1). These were confirmed by the areas under the curve occupied by the various parameters (GR=0.999, PCO=0.994, GPx=0.989, PCO/SOD=0.941, glucose=0.517, HCO3=0.503, HCO3/glucose=0.476, SOD=0.199 at 95% confidence interval) (Table 4).
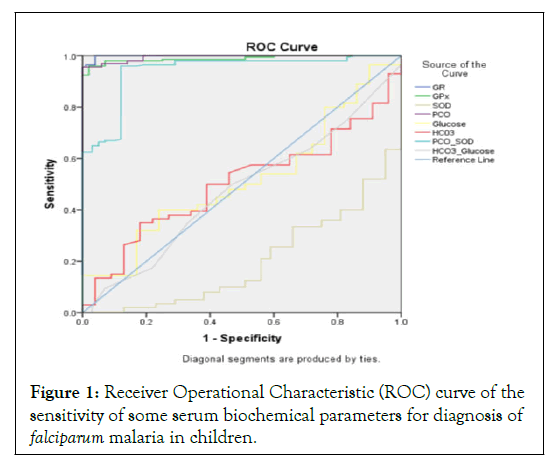
Figure 1: Receiver Operational Characteristic (ROC) curve of the sensitivity of some serum biochemical parameters for diagnosis of falciparum malaria in children.
Note: GR=Glutathione Reductase; GPx=Glutathione Peroxidase; SOD=Superoxide Dismutase; PCO=Protein Carbonyl; HCO3=Bicarbonate.
| Parameters | Area under curve | Standard error | Asymptotic significance | Asymptotic 95% confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| GR (U/l) | 0.999 | 0.001 | 0 | 0.997 | 1 |
| GPX (U/l) | 0.989 | 0.005 | 0 | 0.979 | 0.998 |
| SOD (U/ml) | 0.199 | 0.025 | 0 | 0.149 | 0.249 |
| PCO (nmol/ml) | 0.994 | 0.003 | 0 | 0.989 | 0.999 |
| Glucose (mmol/l) | 0.517 | 0.034 | 0.64 | 0.449 | 0.584 |
| HCO3 (mmol/l) | 0.503 | 0.033 | 0.932 | 0.437 | 0.569 |
| PCO/SOD | 0.941 | 0.014 | 0 | 0.914 | 0.969 |
| HCO3/Glucose | 0.476 | 0.035 | 0.499 | 0.408 | 0.544 |
Note: GR: Glutathione Reductase; GPx: Glutathione Peroxidase; SOD: Superoxide Dismutase; PCO: Protein Carbonyl; HCO3: Bicarbonate.
Table 4: Area under the curve for some serum biochemical parameters for Falciparum malaria diagnosis in children.
Parasite (P. falcipaum) density prediction potentials of GR and GPx
Mathematical model for predicting parasite density using serum GR activity: The serum GR activity and the respective parasite density by microscopy of 100 children with severe falciparum malaria before treatment, 48 hours of treatment and 48 hours after treatment were determined. Using regression analysis, different relationships between the predictor variable (GR) and the predicted variable (parasite density) were tested. The relationship with a strong coefficient of correlation (r ≥ 0.8) and the highest coefficient of determination (R2) was accepted as the valid relationship [15]. The relationships tested include: linear, polynomial and logarithmic relationships. The regression models and graphs were drawn up. Serum GR activity had a strong correlation (r=0.923) with parasite density and the polynomial quadratic regression model (y=b0+b1x+b2x2) had the highest R2 (0.853); indicating that this model has the best goodfit for predicting parasite density using serum GR activity in children (Table 5 and Figure 2).
| Model | Summary | ANOVA | Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | R2 | SS | df | MS | F | p-value | B | Std. error | Beta | T | p-value | |
| R | 0.923 | |||||||||||
| R2 | 0.853 | |||||||||||
| Regression | 12296783.58 | 2 | 6148391.79 | 859.76 | 0 | |||||||
| Residual | 2123895.660 | 299 | 7151.164 | |||||||||
| GR | 6.962 | 0.313 | 1.893 | 22.264 | 0 | |||||||
| GR2 | -0.011 | 0.001 | -1.047 | -12.322 | 0 | |||||||
| Constant | -433.154 | 22.514 | -19.239 | 0 | ||||||||
Note: The independent/predictor variable is glutathione reductase, R=Coefficient of correlation; R2=Coefficient of determination; SS=Sum of Squares; df=degree of freedom; MS=Mean Square; F=F-test; B=Constant.
Table 5: Polynomial quadratic regression model of malaria parasite density and serum glutathione reductase activity in children.
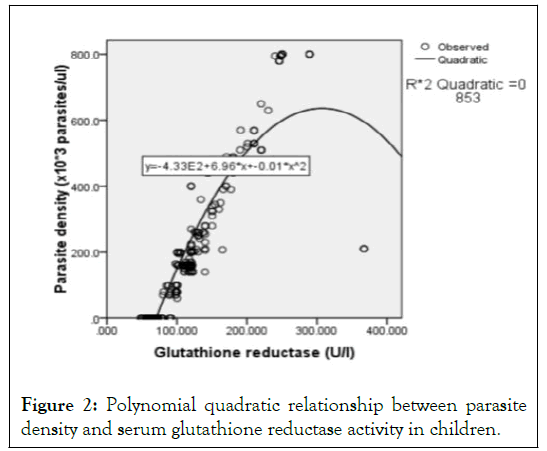
Figure 2: Polynomial quadratic relationship between parasite density and serum glutathione reductase activity in children.
From the general equation for polynomial quadratic relationship:
y=b0+b1x+b2x2
Where:
y=dependent or explained or predicted variable (parasite density); b0=quadratic coefficient; b1=slope; b2=intercept; x=independent/ explanatory/predictor variable (serum glutathione reductase activity).
Thus, from Table 5 and Figure 2:
y=-433.1 + 6.96x +-0.01x2 (1)
R2=0.853
Parasite density (× 103 parasites/μl)=-b0+b1(GR)+b2(GR)2
Parasite density (× 103 parasites/μl)=-433.1+6.96(GR)-0.01(GR)2 (1)
Parasite density (× 103 parasites/μl)=6.96(GR)-433.1-0.01(GR)2 (1)
The polynomial quadratic model means that the rate of change of parasite density depends on the different values of serum GR activity with a cut-off value of >85.78 U/l, which is indicative of the presence of severe malaria in children. From the equation, a unit increase in serum GR activity will cause a 6.96 rise in parasite density [16]. R2 of 85.3% change in parasite density was caused by change in serum GR activity. In other-words, serum GR activity was 85.3% predictive of the actual parasite density in a child. The predicted parasite density value was 85.3% of the actual parasite density.
The above equation holds only when serum GR activity of a child is >85.78 U/l. The normal control range for serum GR activity was 40.30 to 85.78 U/l. Values above 85.78 U/l were predictive of severe malaria (sensitivity of 98.5%; specificity 96.0%; precision of 98.01% at 95% confidence interval) while values <or=85.78 U/l were predictive of absence of severe malaria, thus, parasite density was assumed to be zero at GR activity <or=85.78 U/l.
Comparison of malaria parasite density obtained by microscopy and the mathematical model using serum GR activity
Using the mathematical equation; parasite density (× 103 parasites/μl)=6.96 (GR)-433.1-0.01 (GR)2, the parasite density using the children’s serum GR activity were computed and compared with those of microscopy. They were compared using percentage discrepancy, paired sample t-test (Table 6) and Pearson correlation (Figure 3). The highest percentage discrepancy (24. 26%) was obtained at low parasite range of 1 to 100 × 103 parasites/μl. On the overall, there was no significant difference between the mean parasite densities of both methods (p>0.05). Significant differences (p <0.05) were obtained before treatment among the higher parasite densities (ranges: 201-300; 501-600; 701-800 × 103 parasites/μl) and during treatment at lower parasite density ranges of 1-100 and 101 to 200 × 103 parasites/μl. There was a strong correlation (r=0.927; p=0.000) between the parasite densities obtained by microscopy and the mathematical model [17].
| Parasite density range by microscopy | Parasite density by microscopy Mean ± SD | Parasite density by MM using serum GR activity Mean ± SD | % discrepancy | Paired sample test |
|---|---|---|---|---|
| Day 0 | ||||
| 201-300 (× 103 parasites/µl) | 237.64 ± 27.74 | 352.87 ± 141.45 | 19.54 | p=0.000 |
| 301-400 (× 103 parasites/µl) | 361.69 ± 34.42 | 378.69 ± 69.29 | 2.3 | p=0.432 |
| 401-500 (× 103 parasites/µl) | 462.35 ± 22.03 | 465.44 ± 108.82 | 0.33 | p=0.894 |
| 501-600 (× 103 parasites/µl) | 532.0 ± 21.99 | 581.73 ± 7.35 | 4.47 | p=0.000 |
| 601-700 (× 103 parasites/µl) | 640.0 ± 14.14 | 627.18 ± 17.89 | 1.01 | p=0.672 |
| 701-800 (× 103 parasites/µl) | 793.89 ± 7.96 | 680.39 ± 47.82 | 7.7 | p=0.000 |
| Day 2 | ||||
| 0.0 (× 103 parasites/µl) | 0 | 0 | 0 | ND |
| 1-100 (× 103 parasites/µl) | 83.33 ± 12.81 | 136.70 ± 41.33 | 24.26 | p=0.000 |
| 101-200 (× 103 parasites/µl) | 167.61 ± 20.41 | 227.35 ± 38.89 | 15.13 | p=0.000 |
| Day 7 | ||||
| 0.0 (× 103 parasites/µl) | 0 | 3.72 ± 2.04 | 0 | p=0.084 |
| 1-100 (× 103 parasites/µl) | 85.0 ± 14.43 | 118.32 ± 59.23 | 16.39 | p=0.140 |
| Overall Total (× 103 parasites/µl) | 305.77 ± 271.61 | 324.76 ± 245.65 | 3.01 | p=0.296 |
Note: Day 0=before treatment; Day 2=48 hours of treatment with artesunate; Day 7=48 hours after treatment with artesunate/artemether-lumefantrine combination therapy. ND=No difference between the two zero values.
Table 6: Comparison of malaria parasite density obtained by microscopy and the Mathematical Model (MM) using serum GR activity.
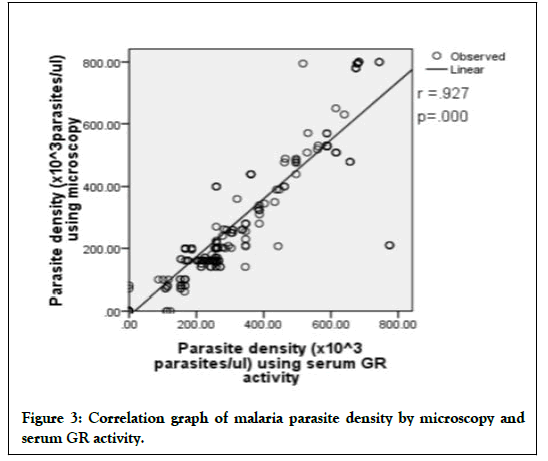
Figure 3: Correlation graph of malaria parasite density by microscopy and serum GR activity.
Mathematical model for prediction of parasite density using serum GPx activity: Using regression analysis, different relationships between the predictor variable (GPx) and the predicted variable (parasite density) were tested. The relationship with a strong coefficient of correlation (r ≥ 0.8) and the highest coefficient of determination (R2) was accepted as the valid relationship. The relationships tested include: linear, polynomial and logarithmic relationships. Serum GPx activity had a strong correlation (r=0.832) with parasite density and the linearlogarithmic regression model (ey=e β1 × β0) had the highest R2 (0.692), indicating that this model had the best good fit for predicting parasite density using serum GPx activity in children (Table 7 and Figure 4) [18].
| Model | Summary | ANOVA | Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | R2 | SS | df | MS | F | p-value | B | Std. error | Beta | T | p-value | |
| R | 0.832 | |||||||||||
| R2 | 0.692 | |||||||||||
| Regression | 9982445.973 | 1 | 9982445.97 | 670.25 | 0 | |||||||
| Residual | 4438233.264 | 298 | 14893.4 | |||||||||
| GPx | 167.527 | 6.471 | 0.832 | 25.889 | 0 | |||||||
| Constant | -593.672 | 31.352 | -18.936 | 0 | ||||||||
Table 7: Linear-logarithmic regression model of malaria parasite density and serum lutathione peroxidase activity in children.
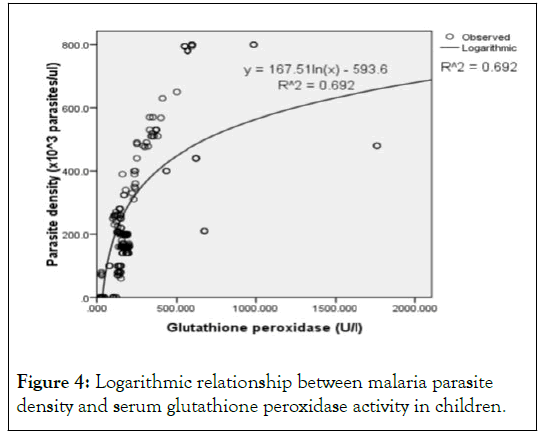
Figure 4: Logarithmic relationship between malaria parasite density and serum glutathione peroxidase activity in children.
Using the general log function:
ey=e β1 X β0
OR
ey=X β0 e β1
Where:
e=natural log;
y=dependent variable;
β1=unknown constant;
β0=unknown impact of x;
x=independent variable.
Using calculus with a simple linear –log model gives:
y=β1+β0Inx
OR
y=β0Inx +β1
Differentiate to obtain:
δy=β0 δx/x
The term on the right-hand side is the percentage change in x while that on the left hand side is the unit change in y.
Thus, from Table 7 and Figure 4:
y=167.51In(x)-593.6 (2)
R2=0.692
Where;
y=parasite density,
167.51=β0=unknown impact of serum GPx activity;
x=serum GPx activity to base e (In),
-593.6=β1=constant,
R2=coefficient of determination.
Thus,
Parasite density (× 103 parasites/μl)=167.51In (GPx)-593.6 (2)
This means that at serum GPx activity >32.51 U/l, 1 percent increase in serum GPx activity increased the parasite density by 167.51 × 103 parasites/μl (β0). This predicted value correctly explained 69.2% (R2, or 0.692) of the actual parasite density in children. This equation applies when serum GPx activity of a child is >32.51 U/l (serum GPx activity reference range in normal children was 6.47 to 32.51 U/l).
Mathematical model for prediction of parasite density using a combination of serum GR and GPx activities: There was a strong correlation (r=0.898) between parasite density and a combination of serum GR and GPx activities, and the linear relationship model (y=B+A1x1+A2x2) had the highest R2 (adjusted R2=0.783) indicating that this model has the best good-fit for predicting parasite density using a combination of serum GR and GPx activities in children (Table 8).
| Model | Summary | ANOVA | Coefficients | 95% C.I | for B Upper | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | R2 | Ad R2 | SS | df | MS | F | p-value | B | Std. Error | Beta | t | p-value | Lower | ||
| R | 0.886 | ||||||||||||||
| R2 | 0.785 | ||||||||||||||
| Adjusted R2 | 0.783 | ||||||||||||||
| Regression | 11317263 | 2 | 5658632 | 541.54 | 0 | ||||||||||
| Residual | 3103416 | 297 | 10449.21 | ||||||||||||
| Constant | -168.66 | 14.94 | -11.29 | 0 | -198.06 | -139.26 | |||||||||
| GR | 2.855 | 0.157 | 0.776 | 18.188 | 0 | 2.546 | 3.16 | ||||||||
| GPx | 0.125 | 0.039 | 0.136 | 3.188 | 0.002 | 0.048 | 0.201 | ||||||||
Note: The dependent/predicted variable is parasite density, The predictors (independent) variables are: GR and GPx, R=coefficient of correlation; R2=Coefficient of determination; SS=Sum of Squares; df=degree of freedom; MS=Mean Square; F=F-Test; B=Constant; CI=Confidence Interval
Table 8: Linear regression model between malaria parasite density and a combination of serum GR and GPx activities in children.
Using the general linear model:
y=B+A1 × 1+A2x2
Where:
y=parasite density;
B=intercept (constant);
A1, A2=slopes (constants);
x1=serum GR activity;
x2=serum GPx activity.
y=-168.66 + 2.855x+0.125x (3)
Adjusted R2=0.783
Thus, from Table 8;
Parasite density (× 103 parasites/μl)=-168.66+2.855(GR) +0.125(GPx) (3)
2.855(GR)-168.66+0.125(GPx) (3)
Every unit rise in combination of serum GR and GPx activities at cut-off values of >85.78 U/l and >32.51 U/l respectively, will in the same order result in 2.855 × 103 parasites/μl and 0.125 × 103 parasites/μl rise in parasite density. The combined predictor variable was able to accurately predict 78.30% (adjusted R2, or 0.783) of the actual parasite density in children at the various cut-off values.
Prompt diagnosis of malaria and parasite quantification is of profound importance for early therapeutic intervention. Various serum biochemical parameters have been used as diagnostic markers for malaria, such as Plasmodium falciparum lactate dehydrogenase, Plasmodium falciparum histidine rich protein 2 etc. However, these markers have their limitations. For example, some parasite strains have been reported to have their HRP2 genes deleted, thereby increasing the chances of false results and they are also limited by their lack of parasite quantification ablility. This therefore necessitates the development of new diagnostic and parasite quantification tools based on novel biomarkers.
In our previous studies, alteration in the concentrations of selected inflammatory markers, oxidative markers, antioxidants, electrolytes and biomolecules in children with severe malaria treated with artesunate/artemether-lumefantrine combination therapy were evaluated, with the aims of determining the response of the parameters to treatment as well as getting novel reliable recovery biomarkers.
In this study, the diagnostic and parasite quantification potentials of selected serum biochemical parameters, which showed fastest and fast responses to treatment in our previous studies, were evaluated in children with severe malaria. Serum GR activity showed the highest diagnostic potential for falciparum malaria in children among all the parameters studied, comparing favourably with microscopy (the gold standard). This was followed by serum GPx activity while other parameters had relatively high sensitivity with relatively low specificity or vice versa, indicating that they are not good malaria diagnostic parameters. The results of this study corroborate earlier findings of Pabon, et al.; and Linares, et al. Who reported that serum GR and GPx activities were increased in children with severe malaria.
For a diagnostic tool to be reliable it must have sensitivity and specificity of >90% and the elevated serum GR activity observed in this study satisfied this condition. In addition, high positive predictive value or precision obtained in this study agrees with high prevalence of falciparum malaria reported in the tropics and implies that early treatment could be initiated in children after effectively diagnosing malaria using serum GR activity. The high sensitivity, specificity, precision and odds ratio of the use of serum GR activity for diagnosis of falciparum malaria in children will reduce the use of antimalarial drugs in malaria negative cases (4% detected in this study) and the number of true malaria cases which are not treated (2% detected in this study). However, use of serum GR activity as malaria diagnostic tool could be limited by the rare hereditary GR deficiency disease. In any case, none of the study children had GR deficiency.
Serum GR activity was reduced significantly (p<0.05) with treatment in such a way that there was no significant difference (p>0.05) between its values on day 7 and that of control (Tables 1 and 2). On day 2, serum GR activities were 64.28U/l, 95.35U/l and 113.40U/l for 0.0 × 103 parasites/μl, 01-100 × 103 parasites/μl and 101-200 × 103 parasites/μl respectively, while on day 7, serum GR activities were 66.3U/l and 93.06U/l for 0.0 × 103 parasites/μl and 01-100 × 103 parasites/μl respectively. This implies and further affirms that serum GR activity could be used as additional tool for monitoring of recovery in children with severe malaria.
In severe malaria, there is parasite induced oxidative stress which depletes some components of the antioxidant system; this leads to increased oxidation of biomolecules such as protein, thus causing an elevation in serum PCO level with a decrease in the Serum Antioxidant (SOD), thus overwhelming the first line enzymic antioxidant defense in an attempt by the host to offset the effect of the increasing oxidative stress. This study revealed normal serum bicarbonate level compared to control and it followed no regular pattern with increase in parasite density (Tables 1 and 2). Thus the buffering capacity of bicarbonate ion in the blood of the children with severe malaria was not adversely affected. Serum glucose concentration of children with severe malaria was within normal range, comparing favourably well with control and it reduced with increase in parasite density. This suggests more consumption of glucose by the parasite at higher parasite density.
Though, serum SOD activity and PCO level were altered in children with severe malaria in this study, corroborating some earlier reports, the association between severe malaria and PCO/SOD, and HCO3/glucose ratios has not been reported in previous literatures to the best of our knowledge and all these parameters exhibited relatively low sensitivity with relatively high specificity or vice versa (Table 3 and Figure 1). This suggests that these parameters may not be effective malaria diagnostic parameters.
Considering the fact that other tests were done to rule out other infections, such as bacterial and viral infections, that could produce similar biochemical changes, the poor malaria diagnostic potential of these parameters could then be due to the influence of other factors such as diet, absorption, excretion, nutritional status, oxidative effect of artemisinins, individual differences in response to artemisinin treatment, level of parasitaemia and state of host immunity.
The mathematical models: parasite density (× 103 parasites/ μl)=6.96 (GR)-433.1-0.01 (GR) 2 and parasite density (× 103 parasites/μl)=167.51 In (GPx)-593.6 were derived for accurate prediction of P. falciparum density using serum GR and GPx activities respectively in children. The results showed that the GR polynomial equation-parasite density (× 103 parasites/ μl)=6.96(GR)-433.1-0.01 (GR) 2 had 85.3% coefficient of determination (R2). There was a strong positive correlation (r=0.927; p=0.000) between the parasite density obtained by microscopy and the GR mathematical model. On the overall, there was no significant (p>0.05) discrepancy between parasite density obtained by microscopy and the mathematical model but significant discrepancies (p<0.05) were observed at some of the lower and higher parasite density ranges. Parasite density obtained using this model predicted P. falciparum density better than GPx linear-logarithmic regression model-parasite density (× 103 parasites/μl)=167.51 in (GPx)-593.6, which had 69.2% as coefficient of determination. The linear equation, parasite density (x 103 parasites/μl)=2.855 (GR)-168.66+0.125 (GPx) obtained on combination of the serum activities of both GR and GPx had 78.30% as coefficient of determination (adjusted R2, or 0.783).
GR reduces oxidized GSH (GSSG) to GSH and GPx oxidizes GSH to oxidized GSH (GSSG) in the presence of β- Nicotinamide Adenine Dinucleotide Phosphate (NADPH) which is oxidized to NADP+. Severe malaria caused an elevation in serum GR and GPx activities as was also reported by Pabon, et al.; and Linares, et al. and these enzymes have high sensitivity, specificity and precision for diagnosis of severe Falciparum malaria in children. However, the use of these enzymes for estimation of malaria parasite density remains to be elucidated.
From this study, the polynomial quadratic equation; parasite density (× 103 parasites/μl)=6.96 (GR)-433.1-0.01 (GR) 2 was derived by relating parasite density as a predicted variable to serum GR activity as a predictor variable and it had its coefficient of determination (R2) as 85.3% (Table 5 and Figure 2). The polynomial quadratic equation means that the rate of change of parasite density depends on the different values of serum GR activity at values >85.78 U/l and as serum GR activity increases, the parasite density will increase in double fold. Also, from the equation, a unit increase in serum GR activity will cause a 6.96 ×103 parasites/μl rise in parasite density (and 85.3% (R2) change in parasite density was caused by change in serum GR activity.Precisely, the parasite density-GR equation holds true only when serum GR activity of a child is >85.78 U/l. From this study, the normal range for serum GR activity was 40. 30 to 85.78 U/l (Table 2); values greater than 85.78 U/l were diagnostic of severe malaria while values less than or equal to 85.78U/l were diagnostic of absence of severe malaria (sensitivity of 98.5%; specificity 96.0%; precision of 98.01% at 95% confidence limit) (Table 3). Thus, parasite density was assumed to be zero at serum GR activity ≤ 85.78 U/l. This implies that this equation can be used to accurately predict 85.3% of the actual parasite density in children and is more predictive than GPx activity (Table 7 and Figure 4) and a combination of both enzymes (Table 8) that respectively predicted 69.2% and 78.30% (R2) of the actual parasite density correctly.
Importantly, there should be a significant agreement and correlation between a reliable potential new method and the gold standard. From this study, there was a strong positive correlation (r=0.927; p=0.000) between the parasite density obtained by microscopy (gold standard) and the GR mathematical model (Figure 3). Overall, there was no significant (p>0.05) discrepancy between the parasite density obtained by microscopy and the mathematical model but significant discrepancies (p<0.05) were observed at some of the lower and higher parasite density ranges (Table 6). These discrepancies may be due to already established errors inherent with microscopy at low parasitaemia, as it is influenced by chance because of low number of fields containing parasites. Also, at high parasitaemia, though microscopy is less influenced by chance, it is highly subjected to human inconsistency due to the tediousness of counting the numerous tiny parasites. Furthermore, peripheral parasite count may not be a true representation of the parasite load, especially due to sequestration common in P. falciparum malaria. These, however may not have influenced the serum GR activity which is a function of the parasite metabolic rate, which also is a function of parasite density. In addition, the count of relative number of leucocytes applicable in microscopy was absent in parasite density estimation using serum GR activity and is relatively more standardized than the conventional microscopy method. There are no decisions required from the analyst that might introduce subjectivity or bias. This implies that the mathematical model-parasite density (× 103 parasites/ μl)=6.96 (GR)-433.1-0.01 (GR)2, could serve as a valuable auxiliary malaria parasite quantification tool in combination with microscopy and could be useful for prompt and accurate clinical and therapeutic decisions and actions in children with severe falciparum malaria.
In conclusion, findings from this study revealed that serum GR activity had the highest diagnostic and parasite density prediction potentials for severe Falciparum malaria in children, comparing favourably well with the gold standard (microscopy). Thus, serum GR activity may be considered as an effective diagnostic and parasite density prediction parameter for severe falciparum malaria.
This study was carried out in line with the ethics guiding research undertakings on human subjects as approved by the ethical committees of university of Ilorin (reference no. UERC/ASN/2014/013) and Jos University Teaching Hospital (Reference No. JUTH/DCS/ADM/127/XIX/5933). Informed consents of the parent(s) of the children or caregivers for participation and publication of findings were obtained before enrolment, after due explanation of the aims and procedures of the project.
The authors declare that there is no competing interest.
There was no grant support.
All data generated or analysed during this study are included in this published article and its supplementary information files.
We acknowledge the tremendous assistance of the staff of chemical pathology laboratory and paediatrics department of Jos university teaching hospital.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Okoli CA, Igunnu A, Adebayo JO, Oguche S, Malomo SO (2023) Malaria Diagnostic and Parasite Density Prediction Potentials of Some Serum Biochemical Parameters in Children with Severe Falciparum Malaria Treated with Artesunate/Artemether-Lumefantrine Combination Therapy. Pediatr Ther. 13:515.
Received: 14-Mar-2023, Manuscript No. PTCR-23-22136; Editor assigned: 16-Mar-2023, Pre QC No. PTCR-23-22136 (PQ); Reviewed: 30-Mar-2023, QC No. PTCR-23-22136; Revised: 22-May-2023, Manuscript No. PTCR-23-22136 (R); Published: 29-May-2023 , DOI: 10.35841/2161-0665.23.13.515
Copyright: © 2023 Okoli CA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.