
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)Volume 10, Issue 6
Chronic urticaria is highly prevalent in the population and a major motive for consultation in general practice as well as in allergology and dermatology services. It represents a heavy burden on patient’s quality of life and results in significant costs. This review article presents a comprehensive analysis of available literature on the definition, classification, pathogenesis, diagnosis and management of patients suffering chronic urticaria and angioedema and provides clues for clinicians taking care of them. Recommendations to utilize the management strategies contained in the guidelines and the instruments to assess severity, control and quality of life are strongly encouraged.
Angioedema; Biologicals; Chronic urticaria; Omalizumab; Small molecules
Chronic Urticaria (CU) is a skin condition highly prevalent worldwide, with prevalence rates ranging between 0.1 and 1.5% in different regions [1]. Severe CU represents an important burden limiting the Quality Of Life (QoL) of affected patients and their families [2], and has important consequences in health systems from direct (medical consultations, medications, laboratory studies) and indirect costs (absenteeism, presenteeism) [3]. Table 1 summarizes the impact of CU on QoL. This review presents an update of the current state of the art for the classification, pathogenesis, diagnosis, and present and future treatments being pursued for chronic severe CU resistant to standard therapy with antihistamines.
| Quality of Life parameters |
|---|
| High costs |
| Presence of comorbidities |
| Unpredictability of symptoms |
| Impact on the family |
| Interference with health related quality of life (HRQoL) |
| School and work decreased performance |
| Resistance to the treatment |
| Long disease duration |
| Effects of concomitant angioedema (body deformation, asphyxia) |
| Interference with sexual function |
| Interference with social interactions |
Table 1: Impact of chronic urticaria on patient’s quality of life.
This paper is a comprehensive review from Pubmed/MEDLINE articles published between January 1st, 2000 and September 30th, 2020 with the following headings: Urticaria, chronic urticaria, angioedema, Omalizumab, small molecules, biological treatments, urticaria guidelines. Original articles, case reports, and review papers were included.
Definition and classification of urticaria
Urticaria can be classified into three clinical phenotypes based on its duration (acute or chronic) and in the presence or absence of inducing factors (inducible versus spontaneous) (Figure 1). Chronic Spontaneous Urticaria (CSU) (idiopathic urticaria), refers to recurrent urticaria lasting for more than 6 weeks that occurs in the absence of an identifiable environmental provoking factor [4]. CSU is the most frequent form of CU, whereas Chronic Inducible Urticaria (CIndU) accounts for approximately 15% of all CU patients. Both, CSU and CIndU may coexist in some CU patients [5].
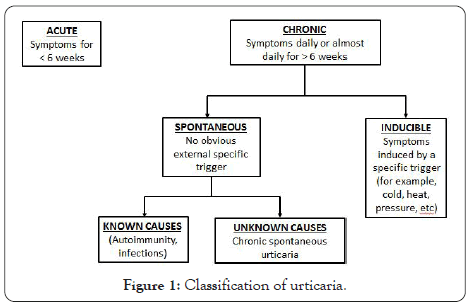
Figure 1: Classification of urticaria.
CSU presents exclusively with wheals in approximately 40% of patients, wheals plus Angioedema (AE) in 40% and as isolated angioedema in up to 20% of cases [6].
CIndU is provoked by physical factors acting on the skin and includes cold urticaria, heat urticaria, symptomatic dermographism, delayed pressure urticaria, pressure urticaria, cholinergic urticaria, solar urticaria, vibratory angioedema, and contact urticaria [7].
Pathogenesis
Most investigators in the area agree that urticaria is a mast cell-driven disease where dysregulation of Mast Cells (MCs) and basophils with subsequent activation and the release of inflammatory mediators in the skin results in the formation of wheals and AE. Other cell types such as eosinophils, T and B lymphocytes, epithelial and endothelial cells also participate [8]. Skin MCs can be activated through multiple pathways since they are complex cellular elements with a myriad of membrane receptors, for example high affinity IgE receptors (FcERI), anaphylatoxin (C5a) receptors, CRTh2 for PGD2, MRGPRX2 for neuropeptides, or proteases and cationic proteins (like Major Basic Protein (MBP) and Eosinophil Cationic Protein (ECP), cKit for Stem Cell Factor (SCF), cytokine receptors (IL-4Rα, Thymus Stromal Lymphopoietin Receptor (TSLP-R), toll-like receptors recognizing PAMPs and DAMPs , and inhibitory receptors such as Siglec-8. The coagulation pathway is also involved in MC degranulation through Protease-activated Receptors (PAR) with thrombin and D-dimer formation.
Additional mechanisms implicated in the pathogenesis of CU are dysregulation of intracellular signaling pathways within MCs and basophils, and autoimmunity related to the production of IgG autoantibodies to FcERI or to IgE (autoimmunity type IIb), or the participation of IgE autoantibodies directed to autoantigens such as Thyroid Peroxidase (TPO), double stranded DNA and IL-24 (autoimmunity type I or autoallergy). Histamine, tryptase, leukotrienes and cytokines released from activated MCs result in sensory nerve stimulation, vasodilatation, and plasma extravasation as well as cell recruitment to the urticarial lesions [9].
Several potential biomarkers for urticaria with a good clinical correlation have been described, including total serum IgE, C-reactive Protein (CRP), the Autologous Serum Skin Test (ASST), anti-TPO IgG autoantibodies and cytokine (IL-17, IL-31, IL-33) serum levels [10-14] (Table 2).
| Biomarker | Clinical correlation |
|---|---|
| Total serum IgE | Higher basal levels correlate with better response to omalizumab and shorter time to relapse |
| C-reactive protein | Higher levels correlate with increased disease activity and shorter duration |
| Autologous serum skin test | Increase disease severity |
| Anti-TPO IgG autoantibodies | More prolonged disease |
| Cytokine (IL-17, IL-31, IL-33) serum levels | Higher levels correlate with increased disease activity and pruritus severity |
Table 2: Potential biomarkers of chronic urticarial.
Diagnosis
In patients with CSU it is essential to obtain a detailed clinical history that includes duration, severity, contributing factors such as foods, medications, thyroid disease, autoimmune disorders, chronic infections, and rarely malignancies. Also, inducible urticaria should be considered including dermographism, cold urticaria, heat urticaria, exercise-induced urticaria, contact urticaria, delayed pressure urticaria and cholinergic urticaria. The response to previous therapies should be inquired.
The EAACI/EDF/WAO 2018 Guidelines recommend limited diagnostic testing unless the history provides clues to an underlying cause [9]. There are validated instruments that permit to quantify CSU severity, control and QoL. Those include the Urticaria Activity score in 7 days (UAS7), the Angioedema activity score, the Urticaria Control Test (UCT), the Urticaria quality of life and AE quality of life tests [15].
Skin tests and in vitro tests to aeroallergens and foods are usually inappropriate unless the history suggests concomitant allergic rhinitis and/or asthma or food allergy. In patients with isolated AE it is mandatory to differentiate histaminergic from bradykinin- mediated AE using algorithms recommended in International Guidelines [9].
Current and future therapies for the management of chronic urticaria
General measures: Some general measures are recommended for patients with CU. Patient questioning and physical examination may help to reveal triggering factors and, when identified, those should be avoided. They include physical agents, drugs (NSAIDs, ACE inhibitors), foods, other allergens, contactants and emotional stress. Concomitant infections require adequate treatment, and other comorbid conditions such as autoimmune thyroid disease, hypertension and metabolic syndrome should be treated [16].
Up to 40% of patients with CSU experience urticarial exacerbations when receiving aspirin or NSAIDs [17], and if NSAID hypersensitivity is suspected from the history or proven by means of drug provocation tests, NSAIDs are to be avoided. If concomitant inducible urticaria is present it has to be treated accordingly [16]. Additional measures to decrease pruritus and patient discomfort include the utilization of physical devices, for example cold compresses.
Pharmacological treatment
The pharmacologic treatment of CU has been delineated in National and International Guidelines that propose a stepwise protocol based on symptom severity and the response to the medications [9,18,19] (Figure 2). There are some differences between International and North American Guidelines [20].
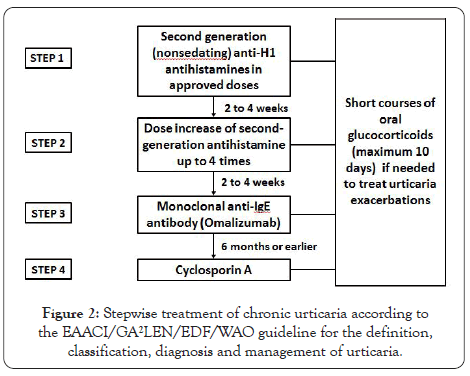
Figure 2: Stepwise treatment of chronic urticaria according to the EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria.
Antihistamines: Nonsedating anti-H1 antihistamines are the first line of pharmacological treatment (Step 1). The North American Position Paper includes first generation (sedating) antihistamines in Steps 2 and 3 [20], although its use is not recommended in the International Guidelines due to their adverse effects (sedation, sleepiness) and because they are not more effective than second generation antihistamines [9].
If the patient does not respond to conventional doses of nonsedating antihistamines, doses can be increased up to 4 times (Step 2). Even at increased doses antihistamines may not be effective in 40%-45% of CU patients [21].
Monoclonal anti-IgE: The only biological medication currently approved for patients with antihistamine-refractory moderate to severe CSU is Omalizumab (OMA), a monoclonal antibody directed to the CE3 domain of human IgE heavy chain, the same site that binds to the FcERI on MCs and basophils. Efficacy and safety of OMA in CSU have been demonstrated in double-blind placebo-controlled studies [22-24], and confirmed by means of meta-analysis of randomized clinical trials [25].
Mechanisms of action of OMA in CSU
Cyclosporin A: Cyclosporin A (CsA) is the second alternative drug effective in patients with treatment-resistant CSU [26,27]. CsA is a calcineurin inhibitor that inhibits T helper cells by blocking the production of inflammatory cytokines. The complex formed between CsA and cyclophilin inhibits the phosphatase activity of calcineurin downregulating the transcription of cytokine genes (IL- 2, IL-3, IL-4, TNF-α) and histamine, leukotriene and prostaglandin release by MCs and basophils in vitro and in vivo , also decreasing serum levels of IL-2R, IL-5, and TNF-α (Table 3).
| Reduction of serum IgE levels |
|---|
| Dissociation of IgE-FceRI binding |
| Reduction of IgE receptor numbers on mast cell and basophil membranes |
| Reduction of mast cell/basophil degranulation |
| Reversion of basopenia and improvement of IgE receptor function in basophils |
| Reduction in anti-FceRI and anti-IgE IgG autoantibody activity |
| Reduction in autoantigen IgE autoantibodies |
Table 3: Mechanisms of action of omalizumab in chronic spontaneous urticaria.
Generally, due to safety concerns, CsA is indicated if OMA fails or is not available. Side effects (hypertension, nephrotoxicity, headache, nausea, abdominal pain and infections) can be associated to CsA therapy and close monitoring of blood pressure, kidney function, and CsA levels every 6 weeks is recommended [28].
Other pharmacological alternatives: Various additional drugs and therapeutic approaches have been used for the treatment of CSU, although they are not currently recommended because of lack of efficacy or because there are not controlled studies assessing their efficacy and safety. They include anti-IL-1, anti-TNF, attenuated androgens, azathioprine, beta agonists, camostat mesylate, colchicine, cromolyn, cyclophosphamide, dapsone, doxepin, gold salts, hydroxychloroquine, intravenous immunoglobulins, leukotriene antagonists, methotrexate, miltofosine, mofetil mycophenolate, nifedipine, plasmapheresis, phototherapy, pseudo- allergen free diets, rituximab, sirolimus, sulphasalazine, tacrolimus, theophylline, thyroxine, tranexamic acid, vitamin D, warfarin/ heparin, and whole blood/serum [29].
OMA is the only biological medication currently approved by regulatory authorities in Europe and North America for the treatment of moderate to severe antihistamine-resistant CU. Other biologicals and small molecules are presently under development for this condition are shown in Table 4.
| Mechanism of action | Target | Drugs |
|---|---|---|
| Drugs inhibiting activation signals and Mast Cell numbers | IgE | Ligelizumab |
| IL-25 | - | |
| IL-33 | - | |
| TSLP | - | |
| SCF | - | |
| IL-4 | Dupilumab | |
| IL-5 | Mepolizumab, Benralizumab, Reslizumab | |
| C5a | - | |
| Mas-related G protein coupled receptor X2 | - | |
| Drugs that inhibit intracellular pathways of mast cell activation | BTK inhibitors | Fenebrutinib, LOU064 |
| Syk inhibitors | GSK2646264 | |
| Drugs that silence mast cells through inhibitory receptors | Siglec-8 | Anti-Siglec-8 (Antolimab) |
| CD200R1 | Anti-CD200R1 (Ly3454738) |
Table 4: Biologicals and small molecules currently in investigation for the treatment of patients with chronic spontaneous urticaria.
Other drugs that have encouraged some interest from investigators but have no approval are TNF-α inhibitors, IL-1 inhibitors, anti- CD20 monoclonal antibody (rituximab), anti-TSLP, anti-IL-13, pitrakinra, AMG-317, anti-NK-1R, anti-C5a, anti-β4 integrin, anti- β4β7 integrin, anti-β7 integrin, T-cell costimulation modulator, CRTh2 antagonist, and histamine H4 receptor antagonists (Table 5).
| Clinical picture | Preventive/general measures | First line therapy | Alternatives | Desensitization possible? |
|---|---|---|---|---|
| Dermographism | Trigger avoidance, light clothing, avoid vigorous toweling, skin hydration | NS 2nd generation AHs | Omalizumab, cyclosporine, phototherapy, photochemotherapy LTRAs, narrow-band UV, H2 antihistamines | No |
| Delayed pressure urticaria/AE | Avoidance of static pressures and tight clothing, use soft shoes, | NS 2nd generation AHs | Montelukast, Omalizumab, Dapsone, Sulfasalazine, anti-TNF, Theophylline, oral corticosteroids short term, chloroquine, oral coumarins, IV Ig, methotrexate | No |
| Acquired cold urticaria | Avoidance of cold exposure, carry epinephrine autoinjector | NS 2nd generation AHs | Ketotifen, Montelukast, Omalizumab, Cyclosporin, antibiotics (doxyciclin, penicillin), anakinra, etanercept, H2 antihistamines | Yes |
| Solar urticaria | Avoidance of relevant wavelengths, protective sunscreens and clothing, | NS 2nd generation AHs | Omalizumab, Cyclosporine, IVIg, Afamelanotide (an alpha MSH analog and melanocortin receptor agonist), phototherapy, photochemotherapyplasmaferesis, topical and systemic steroids, LTRAs, chloroquine | Yes |
| Vibratory urticaria/AE | Avoid provocative stimulus, “warming up” before exercising, | NS 2nd generation AHs | Ketotifen | Yes |
| Cholinergic urticaria | Avoid overheating | NS 2nd generation AHs | Omalizumab, b2 adrenergic stimulants/blockers, botulinum toxin, Danazol | Yes, with autologous sweat |
| Aquagenic urticaria | Barrier creams | NS 2nd generation AHs | UV therapy | No |
Table 5: Management of chronic inducible urticaria.
Treatment of physical urticarias: The first preventive measure to manage physical urticaria is the avoidance of the physical trigger. First line pharmacotherapy includes nonsedating antihistamines in conventional or increased doses and as alternatives OMA and CsA [7]. Tolerance induction by means of progressive and controlled long-term exposure to the stimulus provoking the symptoms is possible for cold urticaria, heat urticaria and solar urticaria.
Chronic urticaria is a frequent motive for consultation in general practice, allergology and dermatology services. The condition interferes with normal life and has significant direct and indirect costs.
Recent advances in the understanding of the pathogenesis of urticaria have encouraged investigators to explore new treatments for patients affected by severe refractory disease. Guidelines for the management of CU as well as Patient Reported Outcome measures (PROs) have been introduced in clinical practice allowing to assess severity, control, and impact on quality of life. These guidance’s are useful for a better patient management and are strongly recommended by authors of this manuscript.
In the near future new therapeutic agents, especially biological and small molecules currently being investigated might show improved efficacy and safety and become useful approaches for patients with antihistamine unresponsive disease.
Citation: Sánchez-Borges M, González-Díaz SN, Martell JAO, Rojo I, Zubeldia IJA (2020) Management of Severe Chronic Urticaria: Current and Future Therapies. J Clin Trials. 10:437.
Received: 13-Oct-2020 Accepted: 26-Oct-2020 Published: 02-Nov-2020 , DOI: 10.35248/2167-0870.20.10.437
Copyright: © 2020 Sánchez-Borges M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.