Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Research Article - (2024)Volume 12, Issue 5
Introduction: Down syndrome continues to be the most common chromosomal disorder, about 1 in every 700 babies born. Children with Down’s syndrome are at an increased risk of developing any type of acute leukemia. In particular, they are 150 times more likely to develop Acute Myeloid Leukemia (AML) and are at a 33 times greater risk of developing Acute Lymphoblastic Leukemia (ALL). The overall survival rate of DS with (AMKL) Acute Mega Karyoblastic Leukaemia is 80%-100% compared to only 35% in non-down syndrome, and about 60%-70% in DS with ALL compared to 75%-85% in non-down syndrome. This variation is due to their genetic mutations and that DS are more prone to infections and side effects of chemotherapy.
Methods: With the approval by the institutional research ethics board, the soft and hard medical records of 29 patients with DS and acute leukemia were reviewed, who according to the hospital database were treated at King Fahad specialist hospital Dammam, between January, 2008 and December, 2022. The diagnosis of DS was based on clinical features and a blood cell karyotype.
Results: During the study period, it shows a total of 29 patients with Down syndrome and acute leukemia it had around 60% B cells and 40% AML, female gender (58%) and male (41.4%), with the Median age of DS leukemia being three years. The median age for DS-ALL is five years, while the Median age for DS-AML is two years. The most common cytogenetic defects in B-CELL ALL is, IGH/IGK rearrangement 9 (53%), then hyper diploidy in 29.4%, followed by t (12,21) ETV6/RUNX1 in 17.7%. Sepsis was the most common complication, and it occurred in 66 episodes in our population, with OS of 76.5% and 75% for DS-ALL and DS-AML, respectively, with a 10% relapse rate with no extramedullary relapse cases diagnosed.
Conclusion: DS-leukemia has a distinct host and leukemic blast biology, which necessitates the development of specific treatment approaches, such as specific molecular testing, meticulous supportive care and individualized therapy, to reduce (TRM) Total Respiratory Morbidity, while optimizing survival figures. Further research to evaluate the effectiveness of currently employed supportive care measures, such as IVIG Intra Venous Immuno Globulin infusions and antimicrobial prophylaxis, is necessary to better quantify their benefits vs. potential risks, costs, or resistance. Moreover, assessing the impact of specific supportive care measures on clinical outcomes is necessary to determine how to allocate resources best to improve overall outcomes in children with DS-leukemia.
Acute children with Down Syndrome (DS) are at an increased risk for development of both acute myeloid and lymphoid leukaemia (DS-ML and DS-ALL, respectively). Although DS-ML is highly curable, the prognosis of DS-ALL is relatively poor compared with the excellent outcomes for ALL in children without DS [1]. Although 1% to 3% of children with ALL and 2% to 15% of children with AML have DS relatively little is known about the cytogenetic patterns in DS-associated leukemia, at least compared with the wealth of information on chromosomal and molecular genetic changes in non–DS-ALL/AML.
The current study addressed several aspects of the diagnosis, management and clinical outcomes of DS-leukemia in single institute in Saudi Arabia.
The outcomes of 29 children with down syndrome and acute leukemia, who were treated in King Fahd specialist hospital in Dammam, pediatric hematology/oncology department, between January, 2008 and December, 2022 were retrospectively analyzed. After obtaining the IRB approval, required data extracted from patient’s electronic medical records then computerized using Microsoft excel sheet and revised instantaneously. Computerized data was exported to Statistical Package for Social Sciences (SPSS). Frequency tables were drawn to explore the findings (frequencies, percentages, measures of central tendencies and dispersion and graphics). Cross-tabulation and ANOVA (Analysis of Variance) used to explore the magnitude of diagnostic interval and the most significant factors affecting it, and the percentages. Overall survival and event free survival were illustrated by the Kaplan Meier curves. Data stored in redcap system for confidentiality.
As all during the study period from 2008 to 2021, a total of 29 patients with Down syndrome and acute leukemia were enrolled in this study. 58.6% were B-cell ALL and 41.4% were diagnosed with AML. Among the AML group, the AMKL subtype was diagnosed in 5 cases, 17.2% this study showed female gender predominance with overall 58% were female and 41.4% were males (Female:Male ratio is 1:4), the female predominance continued within B-ALL and AML subtypes with a ratio of 1.13 for the B-cell ALL and 2 for the AML subtype. Our study showed the overall median age for DS leukemia is three years (1-14 years), with a slightly higher median age for the b-cell ALL subgroup, which was five years, but a marginally lower medial age for the AML subgroup, which was two years (1-10 years) Concerning their clinical presentation, most patients presented with pallor and fever, both occurring at 82%, followed by a petechial rash at 48.3% and bony pains and weight loss at less than 30%. On the other hand, 58% were found to have hepatomegaly at diagnosis, while splenomegaly occurred in 41.4%, and only about 10% had lymphadenopathy at diagnosis. Around 50% of the cases gave a history of previously diagnosed congenital heart defects. Other co-morbidities known to be associated with Down syndrome patients occurred infrequently, such as bowel atresia, bronchial asthma, and hypothyroidism, which all appear in about 10%. All our patients had medullary disease at diagnosis, with only one case: 3% had CNS 1, CSF negative but positive MRI leukemic infiltration. None of the cases had testicular involvement or cutaneous leukemic manifestation the initial diagnostic complete blood picture, cytogenetics and molecular data are shown below (Tables 1 and 2).
| Initial CBC and blast number | Median (range) |
|---|---|
| Median white blood cells count (range) | 7.27/mm3 (1.05-154000) |
| Median hemoglobin concentration (range) | 8.1g/dL (3.50-11.7) |
| Median platelet count (range) | 22,000/mm3 (5000–4,76,000) |
| Median peripheral blast | 38% (1%-96%) |
| Median BM blast | 72% (1%-100%) |
Table 1: Initial Complete Blood Count (CBC) picture.
| Molecular and cytogenetics abnormalities | AML (n=12) | ALL (n=17) |
|---|---|---|
| FL3 | 1 (8.3%) | |
| Gain 1q | 1 (8.3%) | |
| t (4,11) | 1 (8.3%) | |
| Trisomy 8 mosaic | 1 (8.3%) | |
| Complex karyotype | 1 (8.3%) | |
| MDS feature | 1 (8.3%) | |
| 16 q deletion | 1 (8.3%) | |
| T (8,21) | 1 (8.3%) | |
| T (12,21) ETV6 RUNX | 3 (17.7 %) | |
| Hyper-diploidy | 5 (29.4%) | |
| 9p deletion | 1 (5.9%) | |
| CRLEF2/Igh+IKZEF1 | 1 (5.9%) | |
| IGH/IGK re-arrangement | 9 (53%) |
Table 2: Cytogenetics and molecular data.
ML subgroup, TAM was diagnosed in 4 cases (33%) all of them belong to the AMKL subtype. A total of 66 episodes of infection were diagnosed (Table 3), half of which were bacterial in nature, 29 episodes of viral infection, and only three episodes diagnosed with documented fungal infection. The most recurrent bacterial infection was due to Staph aureus sp, other types of organisms were isolated (Tables 3 and 4).
| Organism | Number of stages (Total number 66 stages) | Percentage |
|---|---|---|
| Bacterial | 34 | 52% |
| Viral | 29 | 43.5% |
| Fungal | 3 | 4.5% |
Table 3: Types and percentages of infection stages.
| Bacterial organisms | Viral organisms n (%) | Fungal organisms n (%) | |
|---|---|---|---|
| Staph aureus (34.5%) | 10 | RSV 6 (20.7%) | Candida albicans 3 (10.3%) |
| CD toxin 7 (24.1%) | Influenza species, 6 (20.7 %) | ||
| E. coli species (13.8%) | 4 | COVID-19, 5 (17.2%) | |
| Pseudomonas (10.3%) | 3 | Herpes zoster, 3 (10.3%) | |
| Streptococcus species (10.3%) | 3 | Rota virus, 3 (10.3%) | |
| Klebsiella (6.9%) | 2 | CMV 2 (6.9%) | |
| Salmonella (3.4%) | 1 | EBV 2 (6.9%) | |
| Moraxella catarrhalis (3.4%) | 1 | EBV 2 (6.9%) | |
| Campylobacter (3.4%) | 1 | Enterovirus 1 (3.4%) | |
| Acinetobacter (3.4%) | 1 | Rhinovirus 1 (3.4%) | |
| Stenotrophomonas (3.4%) | 1 | ||
Table 4: Types of isolated organisms.
About 80% of the cases were admitted with febrile infective incidents more than three times during their course of therapy with mucositis stage 3 and above occur in nearly one-third of the cases 27.5%. Since 2019, centre has adopted specific supportive policies for patients of Down syndrome leukemia that are associated with a significant reduction in infective episodes, with about 40% of our patients who were diagnosed after that year benefitting from that strategy. IVIG was administered to about 40% of our patients and was used to be given every month and monitored by IGG level. Kaplan Meier curve 1 Prophylactic antibiotics during myelosuppression periods were given to about 40%, Kaplan Meier curve 2 and also 44.8% were covered with prophylactic antifungal Kaplan Meier curve 3 namely fluconazole during myelosuppression (Figures 1-3).
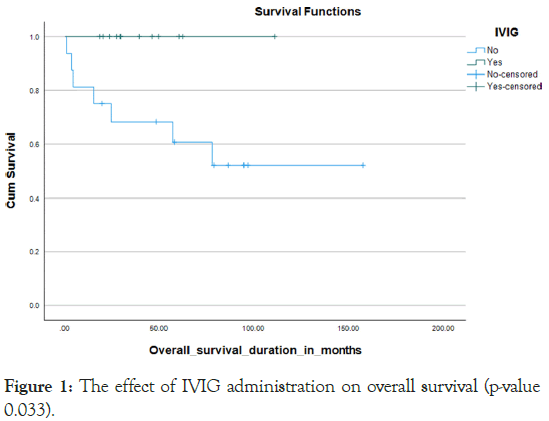
Figure 1: The effect of IVIG administration on overall survival (p-value 0.033).
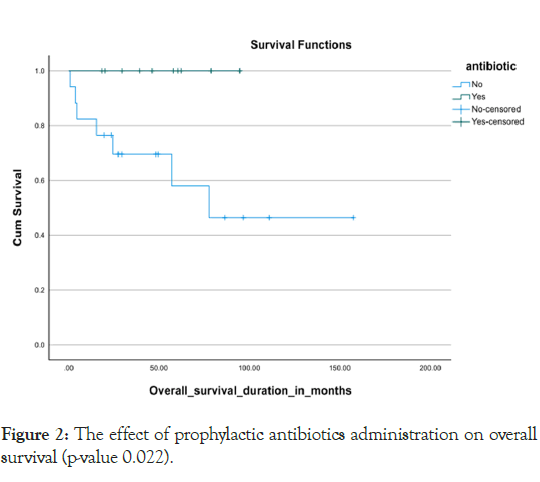
Figure 2: The effect of prophylactic antibiotics administration on overall survival (p-value 0.022).
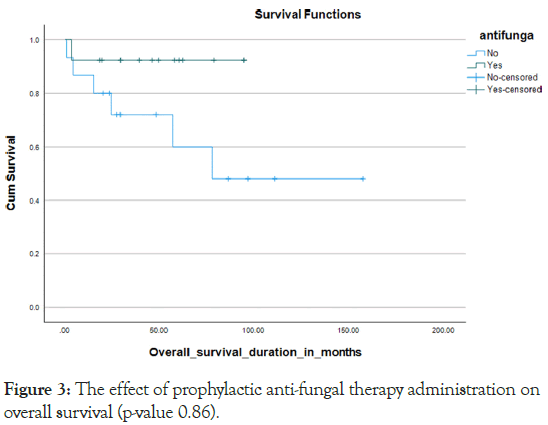
Figure 3: The effect of prophylactic anti-fungal therapy administration on overall survival (p-value 0.86).
Almost all the patients treated with chemotherapy with different protocols (Tables 5). Severe treatment-related morbidities that necessitated Pediatric Intensive Care Unit (PICU) admissions occurred in 45%, with more than 60% needing inotropes or mechanical ventilator support, with the majority of PICU admissions, 84.6%, were due to septic shock 10% were diagnosed with relapsed disease, with 2/3 out of them from the B-ALL subtype and 1/3 belonging to the AML subtype. Almost all our relapse sites were exclusively medullary, with none of them having extramedullary involvement. Seven cases were deceased with a mortality rate of 25%, which included a 23.5% mortality rate for the B-cell all subgroup and a 25% mortality rate for the AML subgroup. We got one case with a missed follow-up. The overall survival for all the leukemia types in DS was 75.8% in 5 years, the 5 years OS for the B-cell ALL and AML subtypes were 76.5% and 75% respectively. (P value 0.77) Kaplan Meier Curve 4,5 however, the survival parameters for the B-cell subtype declined gradually in the following years to reach the range of 63% at 6 years. The 5-year Disease-Free Survival (DFS) for both types together was 86%. The DFS was 79% and 75% for the B-cell ALL and AML subtypes respectively Kaplan Meier Curve 6,7 (p-value 0.786) death occurred during different chemotherapy phases, with 3 cases during the induction phase, 3 patients during the intensification phase and one case during the maintenance phase (Figures 4-7).
| Protocol’s name | Number of patients | Percentage |
|---|---|---|
| CCG 1991 | 11 | 37.9% |
| AALL0932 | 4 | 13.8% |
| AALL1131 | 1 | 3.4% |
| CCG-1891 | 1 | 3.4% |
| BFM 98 | 11 | 37.9% |
| Japanese based DS | 1 | 3.4% |
Table 5: Chemotherapeutic protocols given to patients.
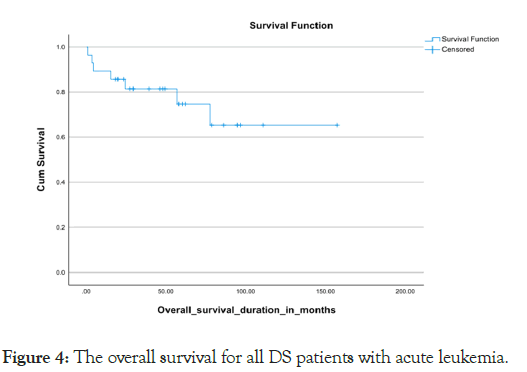
Figure 4: The overall survival for all DS patients with acute leukemia.
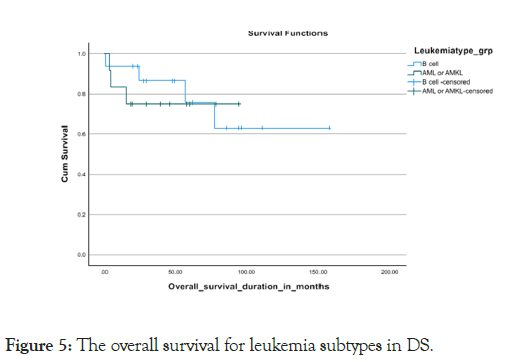
Figure 5: The overall survival for leukemia subtypes in DS.
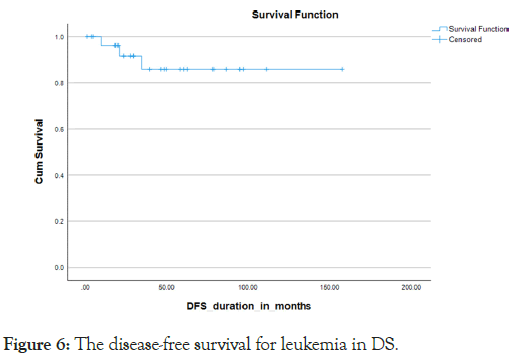
Figure 6: The disease-free survival for leukemia in DS.
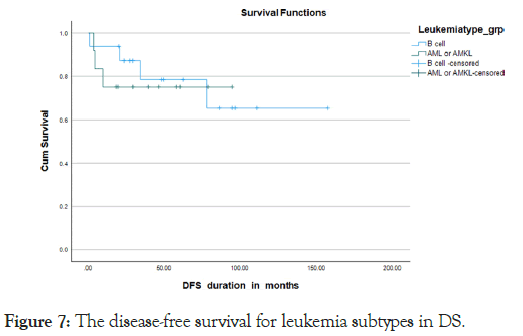
Figure 7: The disease-free survival for leukemia subtypes in DS.
The Saudi consanguinity rate of 56%, with the first cousin type being the most common. These factors lead to a relatively high risk of having offspring with congenital anomalies [2]. In a national study that was conducted in the field over two years (2004 and 2005) is reported that the most standard chromosomal anomaly found in the Saudi Arabia, their survey was Down syndrome (Trisomy 21), with a prevalence of 6.6 per 10,000. Children increased risk of developing significantly Children with Down Syndrome (DS) have both Acute Lymphoblastic Leukemia (ALL) and acute myeloid leukemia [3]. ALL occurs in 300 children with DS versus 1 in 3500 children without DS [4]. T-ALL is only rarely diagnosed as well as infant leukemia, regional study in KSA they reported that a ML-DS represented 15% of all childhood AML in Saudi Arabia. ML leukemia’s are unique and have received a special WHO classification as “Myeloid Leukemia of Down Syndrome”. These are megakaryocytic erythroid leukemia’s that are diagnosed before the age of 5 years and are highly curable with chemotherapy. They are often preceded by a congenital transient pre-leukemic phase called “transient myeloproliferative disorder” or “transient mutation in GATA1 the gene that genetically so matic abnormal myelopoiesis”. Encodes an essential hematopoietic transcription factor occurs in both the transient and the full blown leukemia’s children with Down syndrome had a higher risk of AML before age 5 and a higher risk of ALL regardless of age [5,6].
In children with Down syndrome, ALL was more common between ages 2-4 years, while AML was more common in younger patients-the highest incidence during the first few years of life. For other children, AML incidence remained very low through age 14 years, whereas ALL peaked at age 3 years and steadily declined until age 8 years [7]. similar data from another study showed that the median age of the patients with DS-ALL was 4.8 years, and the sex ratio (male/female) was 1:2, however the median age for patients with DS-ML was 1.8 years, with the AMKL patients having a median age of 1.7 years, and the sex ratio (male/female) was 0:9 our data showed that the Median age of DS leukemia being three years [8]. The median age for DS ALL is five years with sex ratio (male/female) 0:9, while the Median age for DS AML is two years. With sex ratio (male/female) 0:5 Transient Myeloproliferative Disorder (TMD) is identified in approximately 10% of newborns with DS, and it is defined by the presence of megakaryoblasts in the peripheral blood. However, TMD resolves spontaneously in most of the cases (80%), usually in the first 3 months of life, yet 20% to 30% of cases showed transformation into Acute Mega Karyoblastic Leukemia (AMKL) and usually observed before the age of 4 years. and approximately 10% of patients with TAM die from hepatic or multi-organ failure [9]. Mutations in GATA1, occur in almost all the cases with concomitant DS and TMD or AMKL [10]. The current study shows almost one-third of the cases of AML had a history of TMD, all of them having AMKL subtypes which were identified in about 80% of the AMKL group (4 out of 5 cases) The common prognostic genetic subgroups that characterize ALL in children without DS are much less common in DS, such as t (12;21) (ETV6/RUNX1), High Hyper-Diploid (HeH), and trisomies 4 and 10 is uncertain in DS-ALL, on the other hand, even the well-known unfavorable translocations such as t (9;22) and t (4;11) that addressed in patients without DS,these prognostic genetic features have a lower frequency in DSALL [11]. Unfortunately, the current study had some limitations regarding detailed cytogenetics and molecular data. However, the level of the collected data showed that the most common cytogenetic defect in B-CELL ALL is IGH/IGK rearrangement 9 (53%). Hyper-Diploidy in 29.4%, followed by t (12,21) ETV6/ RUNX1 in 17.7%, and no conclusion.
It could be made regarding the cytogenetics and molecular data for DS-ML in our cases as 7 out of the 12 myeloid leukemia cases had different defects and GATA1 mutations testing were not available in our centre. The treatment outcomes for patients with Down Syndrome and Acute Lymphoblastic Leukemia (DS-ALL) have been notably varied compared to patients with Down Syndrome and Acute Myeloid Leukemia (DS-AML). Historical data shows that DS ALL patients have lower survival rates compared to non-DS-ALL patients, and DS patients often experience severe treatment-related side effects, particularly with Me Thotre Xate (MTX). Studies regarding drug sensitivity suggested that DS lymphoblasts do not share the same drug sensitivity patterns as DS AML cells (i.e., lack of increased ARA-C and daunorubicin sensitivity).
This suggests that DS-ALL and AML cells differ biologically, possibly reflecting the lack of GATA1 mutations in DS lymphoblast’s and potential differences in expression of chromosome 21-localized gene [12]. The treatment of patients with DS-ALL is challenging because it is troublesome to discover an adjustment between the dosages of chemotherapy vital to get total long-term remission and those that are exceedingly toxic, individual differences in drug responses complicate matters further. Children with DS-ALL have an adverse outcome compared with non-DS patients due to increased Treatment- Related Mortality (TRM) and a higher relapse rate. Attempts to decrease TRM by introducing reduced treatment intensity regimens may also contribute to the low remission rates in DSALL. Studies tried to determine whether the risk for TRM is related to certain treatment courses or specific chemotherapeutic agents. Currently, conclusions are made regarding the increased risk of mucositis from Me Thotre Xate (MTX), myelosuppression from anthracyclines and hyperglycemia from glucocorticoids again, it is noticeable that the rate of infectious, respiratory, and gastrointestinal [13]. (Adverse events was significantly increased in children with ALL and DS in a recent report, more than onethird (37.5%) of infection-related deaths of children with DS treated for ALL occurred during the low-intensity maintenance phase. Potential causes for this high infection-related mortality in DS include genuine abnormalities of both the innate and adaptive immune system, in addition to what is previously mentioned about the combined myeloid immunosuppressive effect of ALL therapy, hyperglycemia from glucocorticoids, and a higher incidence of mucositis in receiving methotrexate during ALL treatment children with DS. Our study showed 27.5% of the patients had incidents of serious mucositis during their courses of therapy most of them needed introducing TPN during the stages till resolved [14]. Severe Infection episodes that sometimes lead to death occur during treatment for ALL and are higher in children with DS compared to those without DS, and they are observed during all phases of therapy.
One recent study found that more than one-third (37.5%) of infection-related deaths of children with DS treated for ALL occurred intensity maintenance phase-during the extremely feeble infection that necessitated admission to the Pediatric Intensive Care Unit (PICU), providing fluid bolus, use of mechanical ventilation, or administration in the context of severe sepsis and septic shock occurred in nearly half of our study cases (45%) with Inotropes used for 69% of the cases and need for mechanical Ventilator 61.5%, around 84% of the cases admitted to PICU were in a full-blown picture of Septic shock. A similar study by Francesco addressing the Clinical presentation and risk factors of severe infections in children with Down syndrome treated for acute lymphoblastic leukemia showed that the microbiologically documented infection that had clinical presentation as severe infection occurs in 45% of the patients with Staphylococcus, coagulase-negative being the most causative organism isolated in 30% of their cases [15]. Almost the majority of the cases experienced infectious complications, with some of them got different isolated types of organisms more than once or twice, though it was pretty noticeable that the rate and severity of infections decreased significantly after following specific recent supportive care strategies for Down syndrome patients with acute leukemia. The data showed that the most common documented isolated infectious stages were bacterial in nature, with Staphylococcus aureus and Clostridium difficile toxin being the most common offending bacteria, followed by viral infections and to a lesser extent are the fungal infections. It's important to note that the risk of infection-related Treatment-Related Mortality (TRM) is increased even during the maintenance phase. As a result, children with DS-ALL should be monitored more frequently during both the intensive and maintenance periods. Antibiotics should be started at the first sign of infection, even if there is no neutropenia. Protocol amendments for trials AALL0331, which is a study for standard risk B precursor ALL, and AALL0232. A study for high-risk B precursor ALL, include specific recommendations for individuals with Down Syndrome (DS).
These recommendations involve prophylactic antibiotics, hospitalization for febrile neutropenia, leucovorin administration after intrathecal methotrexate, maintenance of immunoglobulin levels higher than 5 g/l, and the administration of stress-dose steroids and/or filgrastim for severely ill patients with DSALL. In the current COG ALL trials, AALL0932, a study for standard risk B precursor ALL, and AALL1131, a study for highrisk B precursor ALL, also require leucovorin administration following intrathecal methotrexate, intensification of therapy based on response during induction in high-risk patients, and the elimination of a second delayed intensification to reduce further treatment-related mortality in children with DS-ALL [16]. Maintenance modifications included administration of vincristine/steroid pulses every 12 instead of every 4 weeks, and equal duration of therapy for boys and girls, on the other hand, regarding ML-DS. The Children’s Oncology Group (COG) AAML0431 trial consisted of 4 cycles of induction and 2 cycles of intensification therapy based on the treatment schema of the previous COG A2971 trial with several modifications. High- Dose ARAC (HD-ARAC) was used in the second induction cycle instead of the intensification cycle, and 1 of 4 daunorubicincontaining induction cycles was eliminated or center adopted specific supportive therapy methods for handling. Since 2019 considering prolonged hospitalization during the Down syndrome leukemia, such as myelosuppression period and a low threshold for admission with febrile illness. It also used monthly IVIG with close monitoring of IGG levels, prophylactic antibacterial ML during-and antifungal antimicrobial coverage, especially for DS myelosuppression periods, and providing less toxic chemotherapeutic regimens. As mentioned earlier, the rates of infections, the severe complications, and the survival figures showed dramatic improvement after strictly adhering to the above policies Our study populations were treated with different chemotherapy protocols as CCG 1991 (37.9%), AALL 0932 (13.8%), AALL 1131 (3.4%), CCG 1891 (3.4%), BFM98 (37.9%) and Japanese based DS used only in one patient (3.4%). In sharp contrast to the excellent prognosis of the myeloid leukemias of DS, the prognosis of children with DS-ALL is significantly worse than children without DS. The 10-year survival of 653 DS-ALL patients enrolled in 16 international prospective therapeutic studies between 1995 and 2005 analyzed in the PdL study was 70% compared with 88% in children without DS. This poor outcome is related to increased relapse risk coupled with increased TRM [11]. JOC in a report from the children's 30 years with DS-patients ages ALL-B, addressing Clinical Oncology (JCO) (n=743) and without DS (n=20,067) who participated in 4 Children's Oncology Group their results showed a more significant number of trials between 2003 and 2019 patients with DS showed Minimal Residual Disease (MRD) ≥ 0.01% at the end of <001) compared to patients without P21.5%. Induction (EOI) therapy (30.8% vs DS. Patients with B-ALL DS demonstrated worse 5-year Event-Free Survival (EFS; 79.2% ± 1.6% vs 87.5% ± 0.3%; p<0001) and overall survival (OS; 86.8% ± 1.4% vs 93.6% ± 0.2%; p<0001) compared to patients without DS in the overall participant sample and in NCI standard-risk and high-risk subgroups. Factors associated with adverse EFS included "age >10 years, white blood count >50 × 103/μL, and end-induction MRD ≥ 0.01%, but not cytogenetics or cytokine receptor-like factor 2 overexpression," as described in the paper [16,17].
Although children with ALL-DS have historically fared less well than their non-ds counterparts (ALL-NDS), recent data indicate that outcomes in ALL-DS are now comparable with ALLNDS with risk-adapted therapies, after adjusting for biological differences between the ALL-DS and ALL-NDS populations [18].
In a retrospective observational study by Schmidt et al., conducted between 2009 and 2018 at "Sf. Maria" Children's Hospital Iasi. It included a group of patients diagnosed with both ALL and AML with and without DS, according to the World Health Organization diagnosis criteria. Their study showed that the OS of DS-AML was 57.1%, which is higher than that of patients with DS-ALL 35.7% and that of AML patients without DS which were diagnosed in the same period in "Sf Maria" Children's Hospital, 45.1% [19]. More improved outcomes result for myeloid leukemia of Down syndrome were published in a report from the Children's Oncology Group concerning the AAML0431 trial in which they found that for the 204 eligible patients, the 5-year EFS was 89.9% and Overall Survival (OS) was 93.0%. The 5-year OS for the patients with refractory/relapsed leukemia was 34.3% [20]. The current study showed almost similar OS of 76.5%, 75% for DS-ALL and DS-AML respectively with 10% relapse rate, with no extra medullary relapse cases were diagnosed.
Although patients with DS-ALL represented an expected 3% of the total population, patients with DS the sample size of 29 patients could be considered a limitation of this analysis. The absence of detailed cytogenetics and molecular data and the fact that this was an institutional study are also critical limiting factors-single. Acute leukemia, need to understand the factorsto improve outcomes for DS a leukemia has-behind treatment failure and TRM. The evidence indicates that DS distinct host and leukemic blast biology, which necessitates the development of specific treatment approaches, such as specific molecular testing, meticulous supportive care and individualized therapy, to reduce TRM while optimizing survival figures. Further research to evaluate the effectiveness of currently used supportive care and administration of IVI G measures, such as antimicrobial prophylaxis to better quantify their benefits vs. potential risks, costs, or resistance. Moreover, assessing the impact of specific supportive care measures on clinical outcomes is necessary to determine how to allocate resources best to improve leukemia. Overall outcomes in children with DS given the relatively small numbers of patients available for study within national groups, international collaboration is essential to develop and test innovative treatment strategies. There is no standard approach to treating relapsed diseases and the function and necessity of being implementation of stem cell transplantation in DS acute leukemia clarified. Future work should include exploring new biomarkers that could predict of the late effects of treatment. On the differ subsequent term-longtoxic effects, and prospective randomized trials focusing on the development of novel, dedicated hand, less toxic treatment regimens that incorporate immunotherapies such as cells should be-blinatumomab, inotuzumab, and Chimeric Antigen Receptor (CAR) T encouraged at the national and international levels.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Awadalla N, Omer H, Alzaki A, Khairy A, Alshafi A, ALdaama S, et al. (2024). Managing Acute Leukemia in Children with Down Syndrome: Clinical Insights and Therapy Results from a Single Institution. J Leuk. 12:399.
Received: 31-Aug-2024, Manuscript No. JLU-24-33769; Editor assigned: 03-Sep-2024, Pre QC No. JLU-24-33769 (PQ); Reviewed: 17-Sep-2024, QC No. JLU-24-33769; Revised: 24-Sep-2024, Manuscript No. JLU-24-33769 (R); Published: 01-Oct-2024 , DOI: 10.35248/2165-7556-24.12.399
Copyright: © 2024 ALdaama S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.