Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Market Analysis - (2019)Volume 6, Issue 3
After the successful completion of the Pharmaceutics 2019. Longdom series, we are pleased to welcome you to the “World Congress on Pharmaceutics and Novel Drug Delivery Systems" The congress is scheduled to take place on September 21-22, 2020 in the beautiful city of Tokyo, Japan. This Pharmaceutics 2020 conference will give you exemplary experience and great insights in the field of research.
After the successful completion of the Pharmaceutics 2019. Longdom series, we are pleased to welcome you to the “World Congress on Pharmaceutics and Novel Drug Delivery Systems" The congress is scheduled to take place on September 21-22, 2020 in the beautiful city of Tokyo, Japan. This Pharmaceutics 2020 conference will give you exemplary experience and great insights in the field of research.
The perspective of the Pharmaceutics 2020 Conference is to set up novel research techniques to help researchers understand how innovative techniques have advanced and how the field has developed in recent years. The pharmaceutical industry channels an important proportion of total research in most developed countries. In Europe, for example, it represents about 13% of total R&D. In recent years, pharmaceutical companies have been accused of devoting their resources mainly to minor improvements over existing medicines that require short clinical trials and have a small risk of not being accepted. The provision functions were an integral part of a large company’s R&D function but nowadays they are most likely to be outsourced under contract to external societies specializing in a particular service, especially if these activities are not seen to offer a competitive advantage to the company.
Drug repositioning is the process of finding new uses of existing drugs, has been gaining popularity in recent years. The availability of several established clinical drug libraries and rapid advances in disease biology, genomics and bioinformatics has accelerated the pace of both activity-based and in silicon drug repositioning. Drug repositioning has attracted particular attention from the communities engaged in anticancer drug discovery due to the combination of great demand for new anticancer drugs and the availability of a wide variety of cell- and target-based screening assays. With the successful clinical introduction of a number of non-cancer drugs for cancer treatment, drug repositioning now became a powerful alternative strategy to discover and develop novel anticancer drug candidates from the existing drug space. In this review, recent successful examples of drug repositioning for anticancer drug discovery from non-cancer drugs will be discussed.
Novel Drug delivery System refers to the formulations, systems and technologies for transporting a pharmaceutical compound in the body as it is desirable to safely achieve its desired therapeutic effects. Drug delivery systems, are those which are mainly based on approaches that are interdisciplinary and that combine pharmaceutics, chemistry, and molecular biology.
In the present scenario the innovative & smart drug delivery technologies are very much important to reach the medicaments to proper site through proper way. In order to deliver the protein, peptide, gene, vaccine, nanoparticles, antigen and some special form of drug – the innovative drug delivery technologies are essential to avoid the enzymatic degradation, acid degradation & some absorption and bioavailability related problems
To enhance the field and make people aware of it. The organizing committee decided to hold a conference. Novel and innovative techniques are the fast growing and developing areas of Drug Discovery and Drug Delivery.
The global biosimilars market is growing at an exponential rate. The CAGR from 2015 to 2020 is projected at over 22%. The biosimilars market is expected to be around $6.2 billion by 2020 from only $2.3 billion in 2015. By the end of this decade the biosimilars would surely occupy 27% of the total pharmaceutical market. Moreover, with the global rise in concern for more accessible-improved- cost effective healthcare, biosimilar drugs would be a more apt choice to the payers, end users, manufacturers over the costly reference biologics. Originator biologics are as costly as about $100,000 per year per patient. Biosimilars on the contrary can be offered at a 30-40% lower price than that of the reference product. However, with all the success stories and opportunities there also lies a sobering 50% failure rate in developing and obtaining license towards marketing of biosimilars. The biosimilars market is categorized into mainly four zones – North America (USA and Canada); Europe(UK, Germany, Spain, Italy, France and Rest of Europe); Asia-Pacific( China, India, Japan, South Korea) and rest of the world ( LATAM and MENA). Key players of the biosimilars market include Amgen, Hospira, Teva, Sandoz International GmbH, Dr. Reddy’s Laboratory, Biocon, Roche, Celltrion, Catalent, Mylan and Merck. There are also certain other companies which are gaining importance in biosimilar developement like Lean Bio Pro-Spain, PPD-USA, SGS Life Sciences-UK and Therapeutic Proteins International-USA. The biosimilars development is mainly concentrated in the therapeutic domains of oncology, blood disorders, autoimmune disorders, endocrine disorders and infectious diseases.
• Biosimilars pharmacoeconomic modelling
• Return on Investment(ROI) for biosimilars
• CAGR of biologics and biosimilars in Europe
• Penetration and uptake of biosimilars in different disease sectors
Pharmaceutical marketing is the last element of an information continuum, where research concepts are transformed into practical therapeutic tools and where information is progressively layered and made more useful to the health care system. Thus, transfer of information to physicians through marketing is a crucial element of pharmaceutical innovation. By providing an informed choice of carefully characterized agents, marketing assists physicians in matching drug therapy to individual patient needs. Pharmaceutical marketing is presently the most organized and comprehensive information system for updating physicians about the availability, safety, efficacy, hazards, and techniques of using medicines. The costs of pharmaceutical marketing are substantial, but they are typical of high-technology industries that must communicate important and complex information to sophisticated users. These costs are offset by savings resulting from proper use of medicines and from lower drug costs owing to price competition.
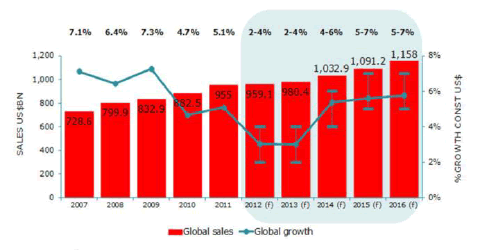
• The global advanced drug delivery market should grow from roughly $178.8 billion in 2015 to nearly $227.3 billion by 2020, with a compound annual growth rate (CAGR) of 4.9%.
• The North American market should grow from nearly $75.7 billion in 2015 to $93.4 billion by 2020, a CAGR of 4.3%.
• The European market should grow from roughly $57.3 billion in 2015 to nearly $72.1 billion by 2020, a CAGR of 4.7%.
• The global systemic antibiotics market should reach nearly $44.7 billion in 2020 from nearly $40.6 billion in 2015 at a compound annual growth rate (CAGR) of 2.0% from 2015 to 2020.
• The beta-lactams market should reach over $22.0 billion by 2020 from over $20.6 billion in 2015, a CAGR of 1.3% from 2015 to 2020.
• The other antibiotic classes market should reach over $10.6 billion in 2020 from nearly $8.3 billion in 2015, a CAGR of 5.0% from 2015 to 2020.
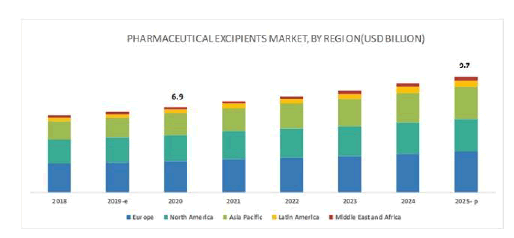
• The global excipients market should reach nearly $6.9 billion by 2020 from over $6.2 billion in 2015, a compound annual growth rate (CAGR) of 1.9% from 2015 to 2020.
• The organic excipients market should reach $6.3 billion by 2020 from nearly $5.8 billion in 2015, a CAGR of 1.7% from 2015 to 2020.
• The inorganic excipients market should reach $433.7 million by 2020 from $351.9 million in 2015, a CAGR of 4.3% from 2015 to 2020.
• The global ophthalmic therapeutic drug market was valued at $12.3 billion in 2014. This market is expected to reach $19 billion by 2020, with a compound annual growth rate (CAGR) of 9.1% from 2014 to 2020.
• The global age-related macular degeneration (AMD) market is expected to grow to nearly $7.9 billion by 2019 from nearly $5.4 billion in 2014, a CAGR of 7.8% from 2014 to 2020.
• The global glaucoma therapeutic market generated revenue of $3.8 billion in 2014, and by 2020 this segment is expected to generate $6.4 billion, with a CAGR of 11.1% from 2014 to 2020.
• Drug Delivery Technology Manufacturers
• Public and Private Physicians
• Healthcare Institutions (Medical Data Centers)
• Research & Clinical Laboratories
• Distributors and Suppliers of Drug Delivery Technologies
• Health Insurance Payers
• Market Research and Consulting Firms
People who have missed attending the past conference are most welcome to present your research ideas at the Pharmaceutics 2020 conference. This conference will help you improve networking with eminent people in the field of research.
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.