
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2023)Volume 14, Issue 2
Introduction: Leishmaniasis is a disease caused by the Leishmania major parasites and afflicted a large number of people every year. This disease is immunologically complex and in some cases, spontaneous improvement is achieved but in some others it enters the systemic phase. Therefore, it seems that the preparation of the vaccine is best suited to combat the disease.
Materials and methods: In this study, Balb/ C mice were vaccinated with new leishmaniasis vaccine and received booster dose one week later. Balb/c mice were challenged 1 week after booster dose. Almost 5 weeks later mice sacrificed. Final stages of preparation of the new L. major vaccine were evaluation of immunologic parameters in three injection groups (LT, LB and LBT) and two injection doses (100, 200 μg/0.1 ml) (totally six groups) post challenge with amastigote which removed from the wound (efficacy) and control group. Efficacy was measured by mice survival rate.
Results and discussion: In this study, the highest IL-4, IFN-γ and CD3+,CD4+, CD8+ and CD25+ were related to the LT group and highest IL-10 and IL-12 were also related to the control group. So it looks like that LT was best injection group and 100 and 200 μg/0.1 ml were best injection doses which recommended for future researches.
Leishmania major; Post challenge; Th1&Th2 cytokines; Spleen’s CDs markers
Many studies about life-cycle, evolution and body immune response to leishmania parasites suggest that an effective vaccine not only must inhibit humoral immune systems, but activates cellular immunity until achieve an effective immune response in such a way that can protect mice and human against cutaneous leishmaniasis. Many studies have so far been conducted on the effects of natural killer cells and on the response to intrinsic immune responses that contribute to understanding the vaccine preparation process [1, 2]. In this connection, many studies have been conducted on the development of cutaneous leishmaniasis vaccine and its immunological responses to the animal model by the author and colleagues. The results of this study provide the final results and finished all of the previous projects [3-12].
In this study, mice received booster doses of new vaccine and one week later challenged with live amastigotes. After almost 5 weeks of post challenging, survival rate and efficacy in mice were measured. The results were analyzed statistically.
Culture and isolation of Leishmania parasites
Leishmania promastigote of the L. major (WHO) strain which were provided by the Tehran University of Medical Sciences. They were grown in NNN medium (Novy-Mac Neal Nicoll) medium supplemented by 5%-10% heat inactivated fetal calf serum. The harvested parasites were washed three times with normal saline solution (0.9%) or phosphate buffer saline (PBS). The parasites were counted in a Neubar chamber and then kept at 70°C till use. After parasite accumulation in one flask, it was diluted to a concentration of 5.92 × 1010. For details of the procedure, please refer to the previous studies by Latifynia et al. [3-12]. The crude antigens prepared from the harvested parasites were diluted to a concentration of 100 and 200 μg/0.1 ml. Based on the previous studies, 100 μg/0.1 ml or 200 μg/0.1 ml Leishmania proteins per dose of the provisional vaccine was selected for the formulation and preparation of the vaccine. The protein content of each dose was estimated by the Lowry method [13]. The vaccine was stored at 4°C until injection. BCG Vaccine (Mycobacterium bovis, Bacillus Calmette Gurine, BCG Strain Pasteur Institute of Iran, Frozen-dried BCG Vaccine Pasteur France, 1173 P2 secondary seed lot C, batch no. 179, Feb. 1995) was suspended and diluted in the SSI solution (125 mg MgSO4, 125 mg K2PO4, 1 mg L-asparagine, 12.5 mg iron ammonium citrate, 18.4 mg 85% glycerol, 0.5 mg citric acid, and 1 ml H2O for injection). The amount of BCG for each injection dose was 2 × 105 CFU/0.1 ml. To prepare the Teucrium polium adjuvant, 400 mg of alcoholic extract of Teucrium polium as dissolved in 1 ml of distilled water; 2.5 mg/0.1 ml was used for each of the injection dosages of the antigen (100-200 μg/0.1 ml) [14]. The adjuvants were added to the leishmania antigen solutions mentioned previously and two injection doses containing 100 μg/ml and 200 μg/ml of antigens are supplemented with adjuvants were prepared.
Experimental design
Forty eight young adult female Balb/c mice were obtained from the Pasture Research Institute. They were randomly assigned to seven polycarbonate boxes for seven treatment groups. All the groups were fed lib with commercial mice chow, had freely across to fresh water and kept in the polycarbonate boxes in a well-ventilated animal room located in the Tehran University of Medical Sciences, Faculty of Medicine. The experimental design consisted of six antigen-injected groups (LT, LB, LBT) either received 100 or 200 μg/0.1 ml and a control group that received no antigen injection. Group LT received 100 or 200 μg/0.1 ml of the crude cocktail antigen preparation plus alcoholic extract of Teucrium polium as adjuvant, Group LB received 100 or 200 μg/0.1 ml of the crude cocktail antigen plus BCG as adjuvant, Group LBT received 100 or 200 μg/0.1 ml of the crude cocktail antigen preparation plus both alcoholic extract of Teucrium polium and BCG as adjuvants. All six injection Groups (LT, LB and LBT) were injected subcutaneously with the antigen preparations at the base of the tail, and they were received similar booster dose one week later. The control group did not receive any antigen injection. Seven days after booster injection all six injection groups challenged with lived amastigote that removed from leishmania lesions from mouse that had experimental leishmania infection. These removed Leishmania amastigote were suspended in 5 ml normal saline, until a thick suspension was obtained. 0.1 ml of this suspension containing valuable amastigotes was inoculated subcutaneously at the base of tail to each mouse for challenge. All of the mice were scarified 4-5 weeks after challenge.
ELISA method
To evaluate cytokine levels in the sera of the animals, at most 2 ml of blood sample was taken from each mouse. The serum was separated by using the routine standard method. The levels of IL-4, IL- 10, IL-12, and IFN-γ in the six injection groups and control group were determined by the sandwich ELISA method according to the recommendations of the manufacturers. Mice serum levels of IL-4, IL-10, IL-12 and IFN-γ in the subjects were measured by using an automated micro plate reader set at 450 nm. The sensitivity limit was 20 µg/ml for IL-4, IL-10 and IFN-γ.
Spleen cell isolation and flow cytometry
Splenic cells and lymphocytes were obtained from Balb/c mice by the collagenase method, Latifynia et al. And in brief, 107 cells were treated with 4 mg/ml proteinase-free collagenase (Sigma-Aldrich, #C6079) for 20 min at 37°C in saline solution at pH 7.5 under gentle agitation, followed by the neutralization of collagenase with an equal volume of complete RPMI media. The cells were centrifuged at 8006 g, re-suspended in saline solution containing 1% BSA, and passed through a 100 µm filter mesh before analysis. Negatively sorted CD4 T cells were obtained at higher than 90% purity according to FACS analysis by cell passage through mouse CD4 subset column kit #MCD4C according to the manufacturer’s instructions (R&D Systems Minneapolis, MN). DNCD3 T-cells were isolated either by depletion of CD4 and CD8T-cells using tandem CD4 and CD8 mouse column kits (#MCD4C and #MCD8C 1000, R&D Systems), or by FACS Aria cell sorter (BD, San Jose, CA) at 98% purity. For purification of DN, DP, and SP4 T-cell subsets, single-cell suspensions were triple-stained with CD3 Ab-FITC, CD4 Ab-PE, and CD8 Ab-Per CP conjugates (BD Phar Mingen, CA) and then FACS-sorted in three simultaneous windows in a FACS Aria instrument. In some experiments, the TCRcd/NK cell depletion of FACS-sorted DNCD3 splenocytes was carried out by the incubation of cells with 2 µg/106 cells of anti-mouse TCRcd Ab-PE (clone #GL3, BD Phar Mingen, San Jose, CA) and 2 µg/106 cells of anti-asialo-GM1Ab-PE conjugates (clone #SH34, ATCC), followed by incubation with anti-PE Abs coupled with magnetic beads and passage through MACS paramagnetic columns according to the manufacturer’s instructions (Miltenyi Biotech Inc., Auburn, CA) [15].
Data analysis and ethics
The data obtained from the experiments were analyzed by using SPSS (SPSS Inc., Chicago, IL, USA). The means were compared by the standard analysis of variance/simple factorial tests and by one-and two-way student Newman Keuls methods. The correlation coefficient analysis was determined by a Pearson average two-tailed test of significance. The study was done in compliance with the Helsinki Declaration, and the protocol was approved by the research deputy of the Tehran University of Medical Sciences, Tehran, Iran.
Almost 5 weeks after challenge, all injected and control group animals weight were measured then blood sample is prepared, spleens removed and weighted. Then were performed all of the following experiments and statistical analysis. In continuing the post challenge experiments, we compared the immune responses of Th1 (IL-12, INF-γ) andTh2 (IL4, IL10) cytokine profiles with cluster determinants (CD4+, CD8+, CD3+ and CD25+) that were induced by L. major amastigotes post challenge. L. major amastigotes were injected in each of these six injection groups (LB, LT and LBT) have received 100 or 200 µg/0.1 ml protein and one booster dose. And also all of the six treatment groups compared with control group. Our results were as follows:
IL-12
The highest amount of IL-12 was related to control, which was not vaccinated and lowest level related to LBT which received both adjuvants (BCG, Teucrium Polium) (Figure 1). The ANOVA test showed that means square of IL-12 compared between two injection doses no considering three injection groups was not significant (P=0.818) (Table 1). Considering IL-12 and multiple comparisons of IL-12 with Tukey’s Honestly Significant Difference (HSD) test and 95% confidence interval IL-12 had not honestly significant mean difference between dose injection 100 and 200 µg/0.1 ml at the 0.05 level (Table 2). And also with ANOVA test means square of IL-12 compared between three injection groups no considering two injection doses had not significant difference (P=0.910) (Table 3). Consider to 2-tailed Pearson correlation test for IL-10 (0.047) and also for CD25+ (0.048) revealed significant correlation whether none of INF-γ, CD3+ and CD8+ had not significant correlation (Table 4). Il-12 and CD3+ had negative and not significant (P=0.104).
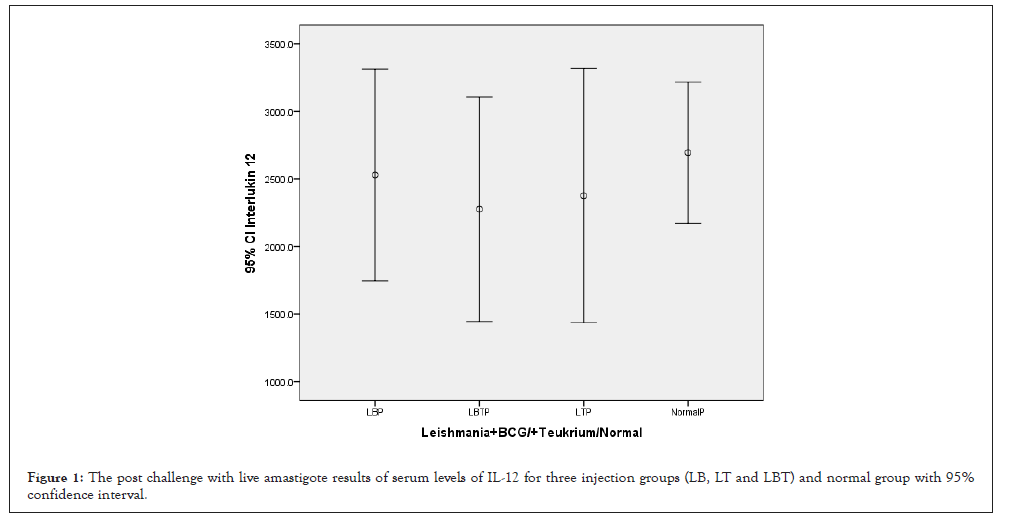
Figure 1: The post challenge with live amastigote results of serum levels of IL-12 for three injection groups (LB, LT and LBT) and normal group with 95% confidence interval.
| Variables | Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| Interlukin 12 | Between Groups | 724529.7 | 2 | 362264.8 | 0.202 | 0.818 |
| Within Groups | 77293418 | 43 | 1797521 | - | - | |
| Total | 78017947 | 45 | - | - | - | |
| Interlukin 4 | Between Groups | 12.577 | 2 | 6.288 | 0.175 | 0.84 |
| Within Groups | 1621.585 | 45 | 36.035 | - | - | |
| Total | 1634.162 | 47 | - | - | - | |
| Interferon gamma | Between Groups | 295.877 | 2 | 147.939 | 0.742 | 0.482 |
| Within Groups | 8973.306 | 45 | 199.407 | - | - | |
| Total | 9269.183 | 47 | - | - | - | |
| Interlukin 10 | Between Groups | 3.694 | 2 | 1.847 | 2.2 | 0.123 |
| Within Groups | 37.776 | 45 | 0.839 | - | - | |
| Total | 41.47 | 47 | - | - | - | |
| Cluster Determinant 3 | Between Groups | 236.71 | 2 | 118.355 | 1.86 | 0.167 |
| Within Groups | 2863.779 | 45 | 63.64 | - | - | |
| Total | 3100.489 | 47 | - | - | - | |
| Cluster Determinant 4 | Between Groups | 136.292 | 2 | 68.146 | 1.316 | 0.278 |
| Within Groups | 2329.974 | 45 | 51.777 | - | - | |
| Total | 2466.266 | 47 | - | - | - | |
| Cluster Determinant 8 | Between Groups | 32.477 | 2 | 16.239 | 2.002 | 0.147 |
| Within Groups | 356.903 | 44 | 8.111 | - | - | |
| Total | 389.38 | 46 | - | - | - | |
| Cluster Determint 25 | Between Groups | 0.078 | 2 | 0.039 | 0.027 | 0.973 |
| Within Groups | 63.959 | 45 | 1.421 | - | - | |
| Total | 64.037 | 47 | - | - | - | |
Table 1: The results of analysis variance (ANOVAs) for serum level IL-12, IL-4, IFN-γ, CD3+, CD4+, CD8+, CD25+ between two injection doses(100 and 200 µg/ml) no considering to three injection groups (LB, LT and LBT) (P<0.01).
| Dependent Variable | (I) Leishmania injection doses 100/200 | (J) Leishmania injection doses 100/200 | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Interlukin 12 | 0 | 100 | 379.1833 | 624.0697 | 0.817 | -1135.71 | 1894.075 |
| 200 | 218.2633 | 624.0697 | 0.935 | -1296.63 | 1733.155 | ||
| 100 | 0 | -379.183 | 624.0697 | 0.817 | -1894.08 | 1135.708 | |
| 200 | -160.92 | 423.9719 | 0.924 | -1190.09 | 868.246 | ||
| 200 | 0 | -218.263 | 624.0697 | 0.935 | -1733.16 | 1296.628 | |
| 100 | 160.92 | 423.9719 | 0.924 | -868.246 | 1190.086 | ||
| Interlukin 4 | 0 | 100 | -0.89548 | 2.77882 | 0.944 | -7.6303 | 5.8393 |
| 200 | 0.16833 | 2.77882 | 0.998 | -6.5664 | 6.9031 | ||
| 100 | 0 | 0.89548 | 2.77882 | 0.944 | -5.8393 | 7.6303 | |
| 200 | 1.06381 | 1.85255 | 0.834 | -3.426 | 5.5537 | ||
| 200 | 0 | -0.16833 | 2.77882 | 0.998 | -6.9031 | 6.5664 | |
| 100 | -1.06381 | 1.85255 | 0.834 | -5.5537 | 3.426 | ||
| Interferon gamma | 0 | 100 | 4.0143 | 6.5368 | 0.813 | -11.828 | 19.857 |
| 200 | -1.2 | 6.5368 | 0.982 | -17.043 | 14.643 | ||
| 100 | 0 | -4.0143 | 6.5368 | 0.813 | -19.857 | 11.828 | |
| 200 | -5.2143 | 4.3579 | 0.461 | -15.776 | 5.348 | ||
| 200 | 0 | 1.2 | 6.5368 | 0.982 | -14.643 | 17.043 | |
| 100 | 5.2143 | 4.3579 | 0.461 | -5.348 | 15.776 | ||
| Interlukin 10 | 0 | 100 | 0.86119 | 0.42413 | 0.117 | -0.1667 | 1.8891 |
| 200 | 0.81024 | 0.42413 | 0.147 | -0.2177 | 1.8382 | ||
| 100 | 0 | -0.86119 | 0.42413 | 0.117 | -1.8891 | 0.1667 | |
| 200 | -0.05095 | 0.28275 | 0.982 | -0.7362 | 0.6343 | ||
| 200 | 0 | -0.81024 | 0.42413 | 0.147 | -1.8382 | 0.2177 | |
| 100 | 0.05095 | 0.28275 | 0.982 | -0.6343 | 0.7362 | ||
| Cluster Determinant 3 | 0 | 100 | -3.2132 | 3.6928 | 0.662 | -12.163 | 5.737 |
| 200 | -6.4942 | 3.6928 | 0.195 | -15.444 | 2.456 | ||
| 100 | 0 | 3.2132 | 3.6928 | 0.662 | -5.737 | 12.163 | |
| 200 | -3.281 | 2.4619 | 0.385 | -9.248 | 2.686 | ||
| 200 | 0 | 6.4942 | 3.6928 | 0.195 | -2.456 | 15.444 | |
| 100 | 3.281 | 2.4619 | 0.385 | -2.686 | 9.248 | ||
| Cluster Determinant 4 | 0 | 100 | 1.5388 | 3.3309 | 0.889 | -6.534 | 9.612 |
| 200 | -2.0593 | 3.3309 | 0.811 | -10.132 | 6.014 | ||
| 100 | 0 | -1.5388 | 3.3309 | 0.889 | -9.612 | 6.534 | |
| 200 | -3.5981 | 2.2206 | 0.248 | -8.98 | 1.784 | ||
| 200 | 0 | 2.0593 | 3.3309 | 0.811 | -6.014 | 10.132 | |
| 100 | 3.5981 | 2.2206 | 0.248 | -1.784 | 8.98 | ||
| Cluster Determinant 8 | 0 | 100 | -2.5319 | 1.4172 | 0.186 | -5.969 | 0.906 |
| 200 | -2.7981 | 1.4172 | 0.131 | -6.236 | 0.639 | ||
| 100 | 0 | 2.5319 | 1.4172 | 0.186 | -0.906 | 5.969 | |
| 200 | -0.2662 | 0.8789 | 0.951 | -2.398 | 1.866 | ||
| 200 | 0 | 2.7981 | 1.4172 | 0.131 | -0.639 | 6.236 | |
| 100 | 0.2662 | 0.8789 | 0.951 | -1.866 | 2.398 | ||
| Cluster Determint 25 | 0 | 100 | 0.0781 | 0.55188 | 0.989 | -1.2594 | 1.4156 |
| 200 | -0.00381 | 0.55188 | 1 | -1.3413 | 1.3337 | ||
| 100 | 0 | -0.0781 | 0.55188 | 0.989 | -1.4156 | 1.2594 | |
| 200 | -0.0819 | 0.36792 | 0.973 | -0.9736 | 0.8098 | ||
| 200 | 0 | 0.00381 | 0.55188 | 1 | -1.3337 | 1.3413 | |
| 100 | 0.0819 | 0.36792 | 0.973 | -0.8098 | 0.9736 | ||
Table 2: The results of a Multiple Comparisons with Tukey HSD for serum level IL12, IL4, IFN-γ, CD3+, CD4+, CD8+, CD25+,between two injection doses(100 and 200 µg/ml) no considering to three injection groups (LB, LT and LBT) (P<0.01).
| Variables | Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| Leishmania injection doses 100/200* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 118125 | 3 | 39375 | 16.5 | 0 |
| Within Groups | 105000 | 44 | 2386.364 | - | - | |
| Total | 223125 | 47 | - | - | - | |
| Interlukin 12* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 908756.7 | 3 | 302918.9 | 0.165 | 0.919 |
| Within Groups | 77109191 | 42 | 1835933 | - | - | |
| Total | 78017947 | 45 | - | - | - | |
| Interlukin 4* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 30.705 | 3 | 10.235 | 0.281 | 0.839 |
| Within Groups | 1603.456 | 44 | 36.442 | |||
| Total | 1634.162 | 47 | ||||
| Interferon gamma* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 290.82 | 3 | 96.94 | 0.475 | 0.701 |
| Within Groups | 8978.364 | 44 | 204.054 | - | - | |
| Total | 9269.183 | 47 | - | - | - | |
| Interlukin 10* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 4.61 | 3 | 1.537 | 1.834 | 0.155 |
| Within Groups | 36.86 | 44 | 0.838 | - | - | |
| Total | 41.47 | 47 | - | - | - | |
| Cluster Determinant 3* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 204.344 | 3 | 68.115 | 1.035 | 0.386 |
| Within Groups | 2896.145 | 44 | 65.821 | - | - | |
| Total | 3100.489 | 47 | - | - | - | |
| Cluster Determinant 8* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 57.895 | 3 | 19.298 | 2.503 | 0.072 |
| Within Groups | 331.485 | 43 | 7.709 | - | - | |
| Total | 389.38 | 46 | - | - | - | |
| Cluster Determinant 4* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 132.406 | 3 | 44.135 | 0.832 | 0.483 |
| Within Groups | 2333.86 | 44 | 53.042 | - | - | |
| Total | 2466.266 | 47 | - | - | - | |
| Cluster Determint 25* Leishmania+BCG/+Teukrium/Normal | Between Groups (Combined) | 3.633 | 3 | 1.211 | 0.882 | 0.458 |
| Within Groups | 60.404 | 44 | 1.373 | - | - | |
| Total | 64.037 | 47 | - | - | - | |
Note: *Correlation is significant at the 0.05 level (2-tailed).
Table 3: The results of analysis variance (ANOVAs) for serum level IL-12, IL-4, IFN-γ, CD3+, CD4+, CD8+, CD25+ between three injection groups (LB, LT and LBT) no considering to two injection doses(100 and 200 µg/ml) (P<0.01).
| Variable | Leishmania injection doses 100/200 | Interleukin 12 | Interleukin 4 | Interferon gamma | Interleukin 10 | Cluster Determinant 3 | Cluster Determinant 8 | Cluster Determinant 4 | Cluster Determinant 25 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Leishmania injection doses 100/200 | Pearson Correlation | 1 | -0.018 | -0.044 | 0.096 | -0.199 | 0.276 | 0.231 | 0.17 | 0.015 |
| Sig. (2-tailed) | - | 0.907 | 0.769 | 0.516 | 0.176 | 0.057 | 0.118 | 0.249 | 0.919 | |
| Sum of Squares and Cross-products | 223125 | -72863.478 | -830.688 | 4366.875 | -604.625 | 7267.281 | 2065.617 | 3982.937 | 56.75 | |
| Covariance | 4747.34 | -1619.188 | -17.674 | 92.912 | -12.864 | 154.623 | 44.905 | 84.743 | 1.207 | |
| N | 48 | 46 | 48 | 48 | 48 | 48 | 47 | 48 | 48 | |
| Interleukin 12 | Pearson Correlation | -0.018 | 1 | 0.05 | 0 | 0.295* | -0.243 | -0.043 | 0.03 | 0.293* |
| Sig. (2-tailed) | 0.907 | - | 0.742 | 1 | 0.047 | 0.104 | 0.781 | 0.841 | 0.048 | |
| Sum of Squares and Cross-products | -72863.478 | 78017947.2 | 17781.938 | 74.36 | 16601.355 | -116107.15 | -7408.56 | 13244.919 | 20330.7 | |
| Covariance | -1619.188 | 1733732.16 | 395.154 | 1.652 | 368.919 | -2580.159 | -168.376 | 294.332 | 451.793 | |
| N | 46 | 46 | 46 | 46 | 46 | 46 | 45 | 46 | 46 | |
| Interleukin 4 | Pearson Correlation | -0.044 | 0.05 | 1 | 0.185 | 0.252 | 0.27 | 0.27 | 0.193 | 0.124 |
| Sig. (2-tailed) | 0.769 | 0.742 | - | 0.209 | 0.084 | 0.064 | 0.067 | 0.188 | 0.402 | |
| Sum of Squares and Cross-products | -830.688 | 17781.938 | 1634.162 | 718.954 | 65.691 | 606.75 | 215.023 | 388.331 | 39.999 | |
| Covariance | -17.674 | 395.154 | 34.769 | 15.297 | 1.398 | 12.91 | 4.674 | 8.262 | 0.851 | |
| N | 48 | 46 | 48 | 48 | 48 | 48 | 47 | 48 | 48 | |
| Interferon gamma | Pearson Correlation | 0.096 | 0 | 0.185 | 1 | 0.066 | -0.079 | -0.082 | -0.039 | 0.148 |
| Sig. (2-tailed) | 0.516 | 1 | 0.209 | - | 0.658 | 0.596 | 0.582 | 0.794 | 0.316 | |
| Sum of Squares and Cross-products | 4366.875 | 74.36 | 718.954 | 9269.183 | 40.671 | -420.902 | -156.416 | -185.402 | 113.803 | |
| Covariance | 92.912 | 1.652 | 15.297 | 197.217 | 0.865 | -8.955 | -3.4 | -3.945 | 2.421 | |
| N | 48 | 46 | 48 | 48 | 48 | 48 | 47 | 48 | 48 | |
| Interleukin 10 | Pearson Correlation | -0.199 | 0.295* | 0.252 | 0.066 | 1 | -0.12 | -0.189 | -0.042 | 0.064 |
| Sig. (2-tailed) | 0.176 | 0.047 | 0.084 | 0.658 | - | 0.416 | 0.204 | 0.778 | 0.666 | |
| Sum of Squares and Cross-products | -604.625 | 16601.355 | 65.691 | 40.671 | 41.47 | -43.052 | -23.842 | -13.379 | 3.29 | |
| Covariance | -12.864 | 368.919 | 1.398 | 0.865 | 0.882 | -0.916 | -0.518 | -0.285 | 0.07 | |
| N | 48 | 46 | 48 | 48 | 48 | 48 | 47 | 48 | 48 | |
| Cluster Determinant 3 | Pearson Correlation | 0.276 | -0.243 | 0.27 | -0.079 | -0.12 | 1 | 0.495** | 0.722** | -0.296* |
| Sig. (2-tailed) | 0.057 | 0.104 | 0.064 | 0.596 | 0.416 | - | 0 | 0 | 0.041 | |
| Sum of Squares and Cross-products | 7267.281 | -116107.152 | 606.75 | -420.902 | -43.052 | 3100.489 | 524.615 | 1996.736 | -132.08 | |
| Covariance | 154.623 | -2580.159 | 12.91 | -8.955 | -0.916 | 65.968 | 11.405 | 42.484 | -2.81 | |
| N | 48 | 46 | 48 | 48 | 48 | 48 | 47 | 48 | 48 | |
| Cluster Determinant 8 | Pearson Correlation | 0.231 | -0.043 | 0.27 | -0.082 | -0.189 | 0.495** | 1 | .677** | 0.303* |
| Sig. (2-tailed) | 0.118 | 0.781 | 0.067 | 0.582 | 0.204 | 0 | - | 0 | 0.038 | |
| Sum of Squares and Cross-products | 2065.617 | -7408.56 | 215.023 | -156.416 | -23.842 | 524.615 | 389.38 | 616.701 | 47.336 | |
| Covariance | 44.905 | -168.376 | 4.674 | -3.4 | -0.518 | 11.405 | 8.465 | 13.407 | 1.029 | |
| N | 47 | 45 | 47 | 47 | 47 | 47 | 47 | 47 | 47 | |
| Cluster Determinant 4 | Pearson Correlation | 0.17 | 0.03 | 0.193 | -0.039 | -0.042 | 0.722** | 0.677** | 1 | 0.223 |
| Sig. (2-tailed) | 0.249 | 0.841 | 0.188 | 0.794 | 0.778 | 0 | 0 | - | 0.127 | |
| Sum of Squares and Cross-products | 3982.937 | 13244.919 | 388.331 | -185.402 | -13.379 | 1996.736 | 616.701 | 2466.266 | 88.737 | |
| Covariance | 84.743 | 294.332 | 8.262 | -3.945 | -0.285 | 42.484 | 13.407 | 52.474 | 1.888 | |
| N | 48 | 46 | 48 | 48 | 48 | 48 | 47 | 48 | 48 | |
| Cluster Determinant 25 | Pearson Correlation | 0.015 | 0.293* | 0.124 | 0.148 | 0.064 | -0.296* | 0.303* | 0.223 | 1 |
| Sig. (2-tailed) | 0.919 | 0.048 | 0.402 | 0.316 | 0.666 | 0.041 | 0.038 | 0.127 | - | |
| Sum of Squares and Cross-products | 56.75 | 20330.7 | 39.999 | 113.803 | 3.29 | -132.08 | 47.336 | 88.737 | 64.037 | |
| Covariance | 1.207 | 451.793 | 0.851 | 2.421 | 0.07 | -2.81 | 1.029 | 1.888 | 1.362 | |
| N | 48 | 46 | 48 | 48 | 48 | 48 | 47 | 48 | 48 | |
Note: *Correlation is significant at the 0.05 level (2-tailed).
**Correlation is significant at the 0.01 level (2-tailed).
Table 4: : The results of Correlations between serum levels of IL-17, IL-23, MW, SW, PSW/MW, NPS, MPS for two injection doses (100 and 200µg/ml) no considering to three injection groups (LB, LT and LBT) (P<0.01).
IL-4
The highest amount of IL-4 was related to LT group and lowest level related to LBT which received both adjuvants (BCG, Teucrium Polium) and control and LB were almost equal together (Figure 2). The ANOVA test showed that means square of IL-12 compared between two injection doses no considering three injection groups was not significant (P=0.840) (Table 1). Considering IL-4 and multiple comparison of IL-4 with Tukey’s (HSD) test and 95% confidence interval IL-4 had not honestly significant mean difference between dose injection 100 and 200 µg/0.1 ml at the 0.05 level (Table 2). And also with ANOVA test means square of IL-4 compared between three injection groups no considering two injection doses had not significant differences (P=0.839) (Table 3). Consider to Pearson correlation with IL-10 two-tailed test had almost significant with (IL-10 (0.084), CD3+ (0.064) and CD8+ (0.067)) whether none of IL12, INF-γ, CD25+ and CD4+ had not significant (Table 4).
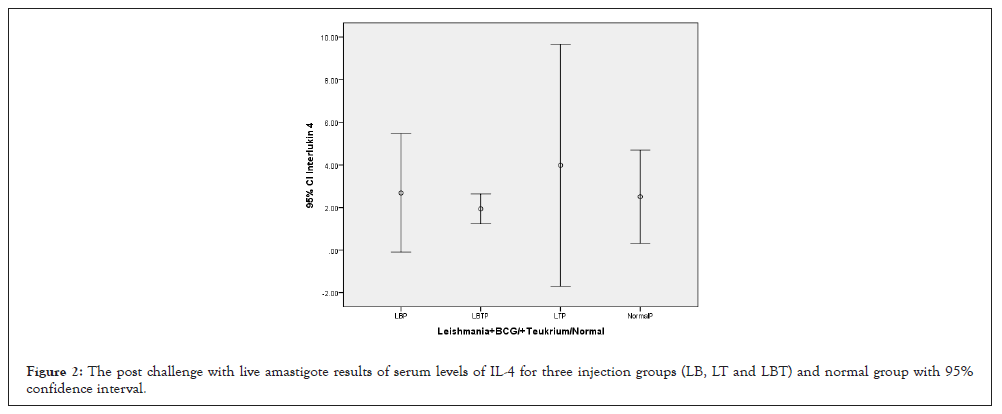
Figure 2: The post challenge with live amastigote results of serum levels of IL-4 for three injection groups (LB, LT and LBT) and normal group with 95% confidence interval.
INF-γ
The highest amount of INF-γ was related to LT group and lowest level related to LB, control and LBT were almost equal together (Figure 3). The ANOVA test showed that means square of INF-γ compared between two injection doses no considering three injection groups was not significant (P=0.482) (Table 1). Considering INF-γ with multiple comparisons of Tukey’s (HSD) test and 95% confidence interval INF-γ had not honestly significant mean difference between dose injection 100 and 200 µg/0.1 ml at the 0.05 level (Table 2). ANOVA test means square of INF-γ compared between three injection groups no considering two injection doses had not significant difference (P=0.701) (Table 3). Consider to Pearson correlation with two-tailed test with IL-4(0.209), CD3+, CD8+, IL12, CD25+ and CD4+ had not significant correlation (Table 4).
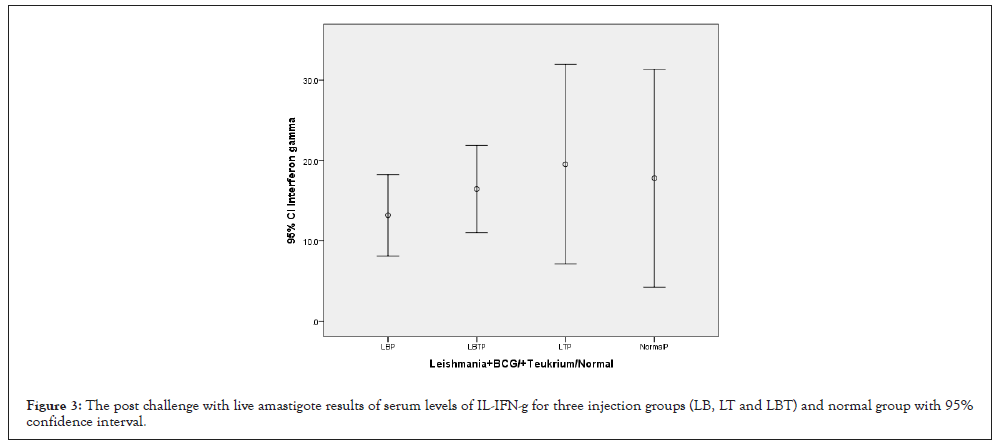
Figure 3: The post challenge with live amastigote results of serum levels of IL-IFN-g for three injection groups (LB, LT and LBT) and normal group with 95% confidence interval.
IL-10
Considering to Figure 4, highest IL-10 was related to control group and lowest to LBT group. Groups LB and LT were almost equal. The ANOVA test showed that the means square of Th2 cytokine (IL-10) between the injection groups and in comparison to another cytokines(IL-12, INF-γ, IL4 and CD4+, CD8+, CD3+ and CD25+) had not significant statistical differences (p=0.123) (Table 1). Multiple comparisons of Tukey’s (HSD) test and 95% confidence interval between dose injection 100, 200 µg/0.1 ml and control group at the 0.05 level showed that IL-10 had not significant mean difference but was more was more significant than the rest (Table 2). ANOVA test means square of IL-10 compared between three injection groups no considering two injection doses had not significant difference (P=0.155) but was remarkable (Table 3). Consider to Pearson correlation with two-tailed test IL-10 had significant with IL-12(P=0.047), almost significant with IL-4(0.084) and CD8+, CD3+, CD25+, CD4+ had not significant correlation (Table 4).
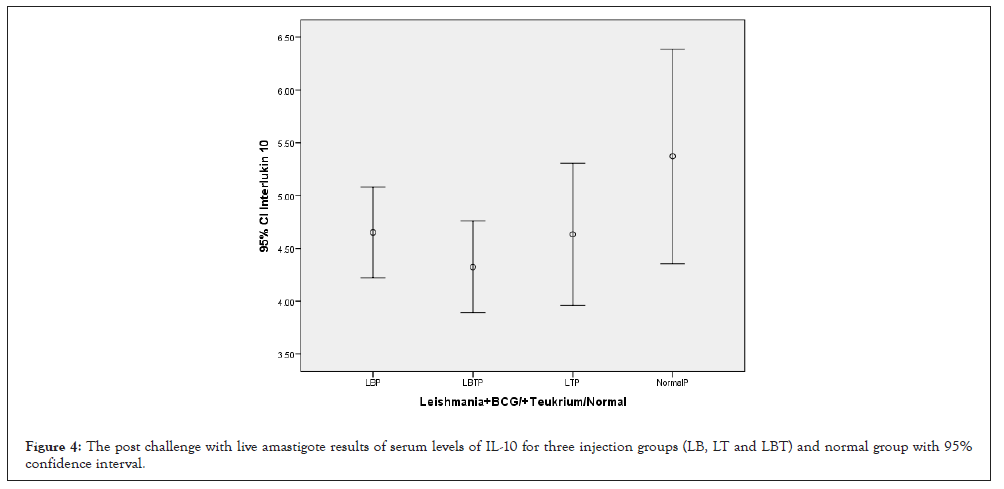
Figure 4: The post challenge with live amastigote results of serum levels of IL-10 for three injection groups (LB, LT and LBT) and normal group with 95% confidence interval
Cluster determinant 3 (CD3+)
Consider to confidence Interval 95% the highest CD3+ was related to LT and lowest related to control group. Groups LB and LBT were almost equal to each other (Figure 5). The ANOVA test showed that the means square of Th2 cytokine (CD3+) between two injection doses and no considering three injection groups had not significant statistical differences (p=0.167) but was remarkable (Table 1). Considering CD3+ with multiple comparison of Tukey’s (HSD) test and 95% confidence interval CD3+ had not significant mean difference between dose injection 100, 200 µg/0.1 ml at the 0.05 level (Table 2). And also with ANOVA test means square of CD3+ compared between three injection groups no considering two injection doses had not significant difference (P=0.386) (Table 3). Consider to Pearson correlation with two-tailed test revealed that IL-12(P=0.104) had not significant but was remarkable, while IL-4(0.064) almost CD8+, CD4+ (P=0.000) had significant and CD25+ (0.041) relatively significant compared to others and none of IFN-γ, IL-10 had not significant correlation (Table 4).
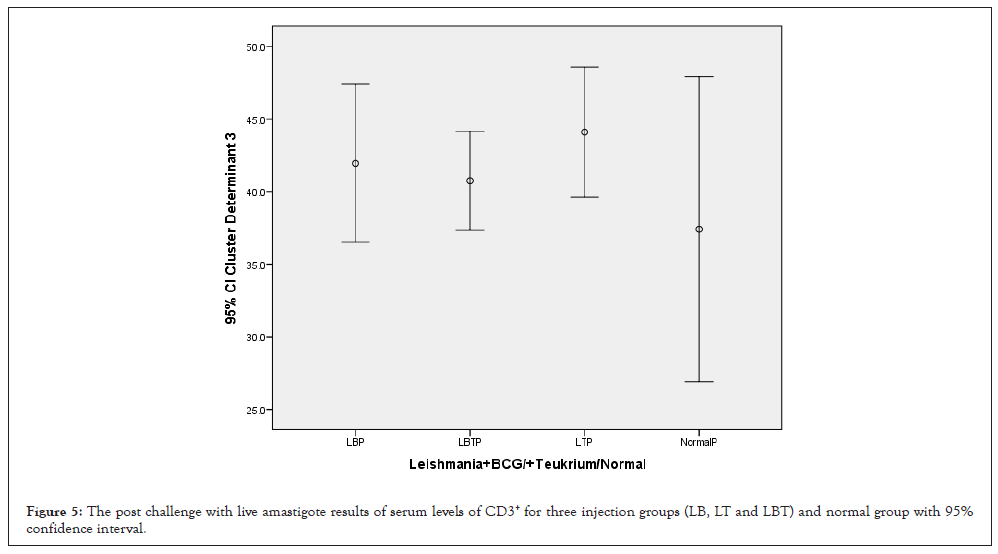
Figure 5: The post challenge with live amastigote results of serum levels of CD3+ for three injection groups (LB, LT and LBT) and normal group with 95% confidence interval.
Cluster determinant 8 (CD8+)
The ANOVA test showed that the means square of (CD8+) between two injection doses no considering three injection groups had not significant statistical differences but was remarkable (p=0.147) (Table 1). The lowest amounts of CD8+ were related to control and LBT and highest amounts related to LT group (Figure 6). Statistical analysis with multiple comparisons of Tukey’s (HSD) test and 95% confidence interval for CD3+ had not showed significant mean difference between dose injection 100, 200 µg/0.1 ml at the 0.05 level (Table 2). ANOVA test means square of CD8+ compared between three injection groups no considering two injection doses had almost significant difference (P=0.072) (Table 3). Consider to Pearson correlation with two-tailed test revealed that CD3+, CD4+, (P=0.000), CD25+ (P=0.038) had significant correlation, while, IL-4(0.067) almost significant, and IL-12, IFN-γ, IL-10 had not significant (Table 4).
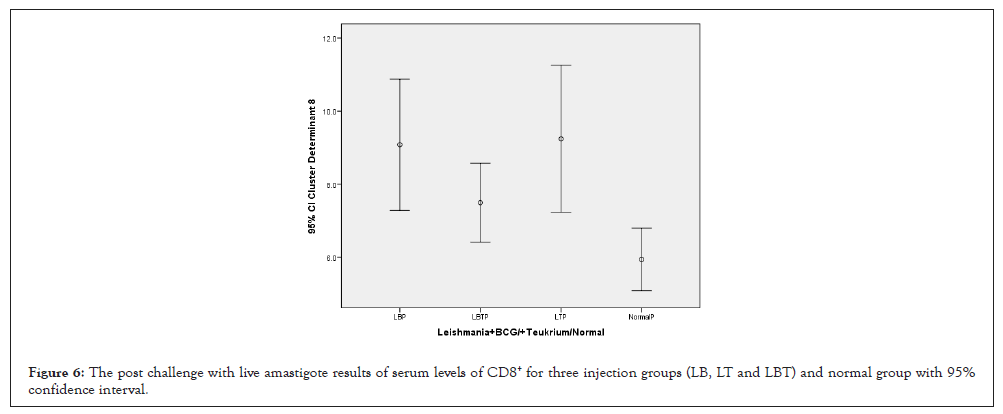
Figure 6: The post challenge with live amastigote results of serum levels of CD8+ for three injection groups (LB, LT and LBT) and normal group with 95% confidence interval.
Cluster determinant 4 (CD4+)
Consider to Figure 7, highest amounts of CD4+ in spleen related to LT and lowest were related to LT and control groups. And with ANOVA test the means square of (CD4+) between two injection doses no considering three injection groups had not significant statistical differences (P=0.278) (Table 1). Statistical analysis with multiple comparisons of Tukey’s (HSD) test and 95% confidence interval for CD4+ had not showed significant mean difference between dose injection 100, 200 µg/0.1 ml at the 0.05 level (Table 2). And also with ANOVA test means square of CD4+ compared between three injection groups no considering two injection doses had not significant difference (P=0.483) (Table 3). Consider to Pearson correlation with two-tailed test revealed significant correlation with CD3+ and CD8+ (P=0.000) and CD25+ (P=0.127) IL-12, IFN-γ and IL-10 and IL-4 were remarkable, but not significant (Table 4).
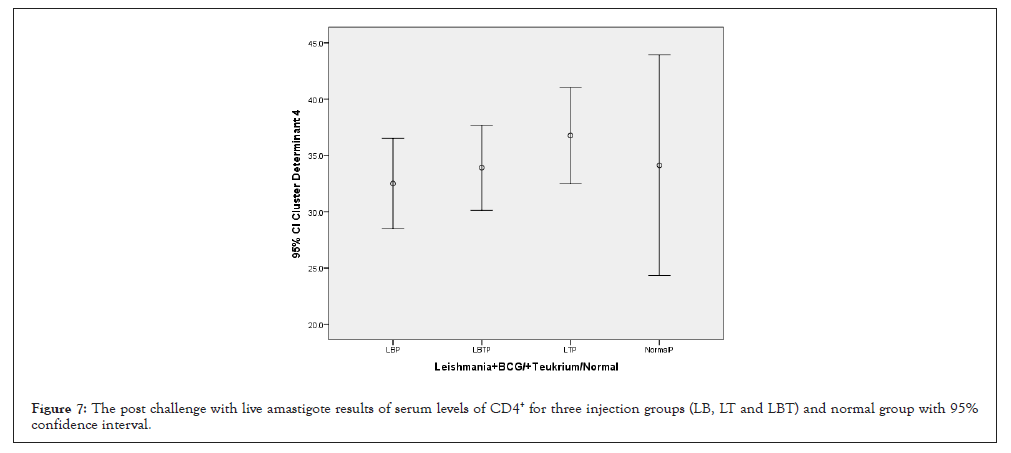
Figure 7: The post challenge with live amastigote results of serum levels of CD4+ for three injection groups (LB, LT and LBT) and normal group with 95% confidence interval.
Cluster determinant 25 (CD25+)
The highest amounts of CD25+ with 95% confidence Interval was related to LT group and lowest amounts related to LB group control and LT group were almost equal (Figure 8). The means square of (CD25+) between two injection doses no considering three injection groups had not significant statistical differences (P=0.973) (Table 1). Statistical analysis with multiple comparisons of Tukey’s (HSD) test and 95% confidence interval for CD25+ had not showed significant mean difference between dose injection 100, 200 µg/0.1 ml at the 0.05 level (Table 2. ANOVA test means square of CD25+ compared between three injection groups no considering two injection doses had not significant difference (P=0.458) (Table 3). Consider to Pearson correlation with two-tailed test revealed significant correlations with IL-12(P=048), CD3+ (P=0.041), and CD8+ (P=0.038), while had not any significant correlation with IL-4, IFN-γ, CD4+ (Table 4).
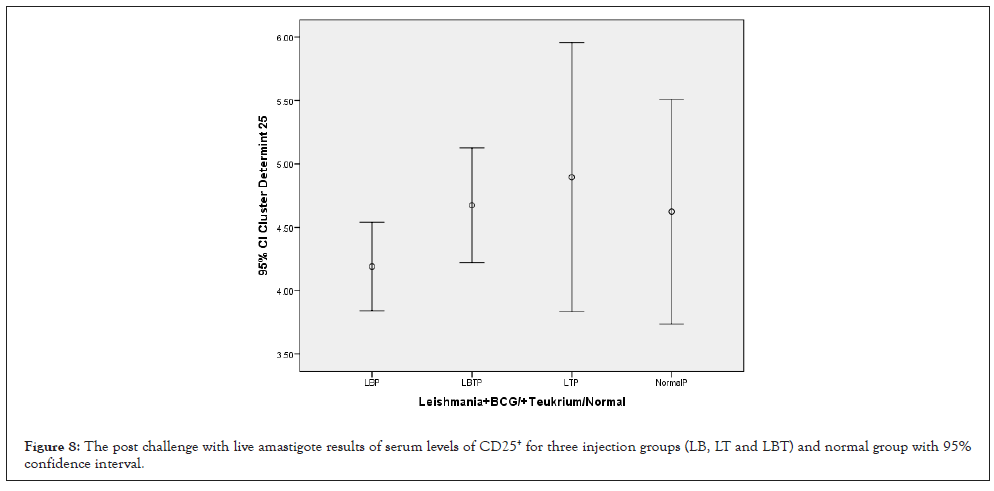
Figure 8: The post challenge with live amastigote results of serum levels of CD25+ for three injection groups (LB, LT and LBT) and normal group with 95% confidence interval.
In present experiment, we are prepared the L. major leishmania vaccine for fourth time. According to this topic, we measured the safety, toxicity several times in different conditions, and confirmed the authors’ previous results (reproducibility). Considering to authors’ previous studies, this time, we used amastigote removed from leishmaniasis ulcers to challenge in mice for the first time. Our current results showed that highest level of IL-4, IFN-γ, CD3+, CD4+, CD8+ and CD25+ were related to LT and the highest level of IL-10 and IL-12 related to control group. The results of LBT were between LT and LB groups. It can be deduced that a high dose may have occurred, because of BCG adjuvant had weekly mycobacterial activity and Teucrium polium as adjuvant increased cellular immunity. Both humoral and cellular immune responses were observed in this group. Conclusion and discussion in various aspects are presented below:
1. Immunogenicity, protective and efficacy were evaluated after safety, toxicity, sterility and endotoxin examination. We choose the best injection group and dose based on previous published articles’ findings and present study on the animal model [3-12]. Consider to this information, the LT group and injection doses 100 and 200 μg/0.1 ml is recommended. This is due to both proper immunological parameters and immunization against promastigote, and the highest survival time for mice in this group. It should be noted that we used isolated amastigote from the wound for challenge for first time.100% of the mice survived for up to 5 weeks, and if further study continued, some groups’ mice still survived. The results of injection doses (100 and 200 μg/0.1 ml) were almost the same.
2. According to our previous studies, and the results of present study, this new vaccine can be stimulates DTH after injection [3-12]. To understand and explain what happened after the injection of this vaccine in live organism (Balb/C mice), we guess, it increased IL-12 and IFN-γ in more steps but decreased IL-10 in recommended group (LT). In some groups which are not recommended, we observed increasing number of spleen’s white pulp and them sizes (site of antibodies synthesis). On the contrary, if these values were low, the result was in favor of the new vaccine. Fortunately, this was seen in some favor groups with our new vaccine [4, 6, 7, 9]. But in some groups that observed either low dose only induces humoral response and or high dose increased induce both humoral and cellular immune responses which is estimated that the synergistic effect on each other. And the both humoral and cellular immune response is not in favor of any cutaneous leishmania vaccine. Anyway, when mice challenged with promistigote, IL-12 and IFN- γ were increased in any group that had more survival. And on the contrary, the high levels of IL-10 were observed in groups that had less survival rate. Of course, in our experiments were some groups that had high IL-12 and also high IL-10 which had high doses had less survival rate after challenge with live promistigote and live amasigote (present study) also [6,8]. Our studies confirmed other studies that reported an increase of IL-12 and IFN- γ for protective immunity and also increase of IL- 10 for non-protective immunity against leishmaniasis disease. In present study we used amastigote which isolated from the wound for challenge. We guess in this challenge, some of the antigens found in the natural cycle of the life of the leishmaniasis (saliva and culture medium promastigote) have been eliminated, or there were antigens that cannot be found in the challenge with promastigote. By doing so, it's possible that some of the antigenic determinants were removed and some others appeared. We suppose that, appeared antigens were necessary for diagnosis by the immune system. Interesting point in our study was used promistigotes to preparation a new vaccine, while we used live amastigote for challenge. Our results after challenge with amastigote was showed that secretion of IL-4 increased in LT-group, while IL-12 and IL-10 increased in control and LB groups. Dendritic cells (DCs) of the antigen presenting cells are potent and can effectively activate the T cells [16]. Previous studies show that DCs are a source of different cytokines such as IL-12, IL-10 and IFN-γ [17-19]. The incubation of Leishmania promastigote with dendritic cells can induce early Il-12 production in vitro, which might have originated from the pre-existing pool of Il-12 p70 which is secreted shortly after the ligation any microbial product, suggest the role of DCs in initiation of T cell immune response in leishmania infection [20]. It has also been reported that uptake of leishmania amastigotes by skin-derived DC induces IL-12 p70 and up-regulates post vaccination immunity against L. major infection. In marked contrast, L. major inhibits IL-12 production in macrophages [21-22]. IL-10 appears to constitute a major regulatory control in an outcome of infection, as a well as the failure to produce IL-12 associated to active form of disease [23]. In present study, there was a safety memory and it seems that dendritic cells in the cutaneous and subcutaneous tissue respond quickly. And as outlined in Articles, the release of IL-4 Inhibits IL-10 produces of dendritic cells and IL-4 has key roles in differentiation of Th0 to Th1 or Th2. Therefore, it is suggested that the new vaccine has been effective and has yet to show the appropriate immune response. Additionally IL-4 led to decreasing in the amount of IL-10, but directly increasing IFN- γ secreted from of dendritic cells [24, 25]. Secreted IFN- γ lead to synthesis of IL-12 and subsequently activation of IL-12 and IFN- γ loop. In our study, IL-4 decreased and IL-10 increased in control and LB group. It is concluded that if IL-10 increase before induction of IL-4 increases, immune response will go to Th-2 immune response. Even if IL-12 secreted from the origin of dendritic cells and IL-12 and IFN- γ loop constitution nevertheless, IL-10 rise again and immune response will go to Th-2 immune response also. So we can conclude that here IL-4 plays a key role. But the highest IL-12 in the control group can be justified in such a way that IL-12 was naturally high in the control samples in our previous study [10]. Group LB which used the BCG (mycobacterium) adjuvant; on the one hand, it immediately activated the skin’s dendritic cells and IFN-γ and 12-loop and subsequently increased IL-12 level. And on the other hand, inflammation in locally infection caused inducing IL-10 before Il-4 induction. However in LT group which had memory of new vaccine, Il-4 synthetized and cytotoxic and natural killer cells that were related to Th1 immune response had created best cellular immune response and we guess it is best new vaccine injection group. It would probably enter other phase of immune response that had CD8+ (cytotoxic) and CD4+ and also, IL-4 that increased the differentiation of Th-0 to Th-1. This pathway inhibits synthesis and increases of cytokines, which is likely to result in the removal of leishmaniasis and recovery loop and delayed type cellular immunity (immune response), which will increase by IL-12 and also IFN-γ and IL-12 loop cycle in continued. However, as it is evident, if susceptible Balb/c mice injected with amastigote which isolated from the wound, probably it will quickly become infected with visceral leishmaniasis and certainly will die 3-4 months or 12 week later. One of the most important findings in this study is increasing of Il-10 in both groups control and LB group that be concluded that immune system will certainly advance to the leishmaniasis disease. In other words, IL- 10 in the difference between immune protectivity and high power of survival rate on the one hand and leishmaniasis and death of mice on the other hand, plays a key role. It means that, if IL-4 is increased, the synthesis of IL-10 is prevented and IL-12 increases, then 90% of cases, recovery will be occurred and protection against leishmaniasis will be achieved. But if IL-4 synthesis does not occur or produced later than IL-10, even if IL-12 is synthesized, the result will be 100% of cases leishmaniasis disease and subsequent death of Balb/c mice will be occurred.
3. The third important dimension of the vaccine was to obtain the optimum dose and injection group. Because of it may be low dose and activate humoral system which causes systemic leishmaniasis and or be high dose that both humoral and cellular immune systems will be active and most probably lead to leishmaniasis and is likely to a systemic muco-cutaneous leishmaniasis.
Fortunately, we reached to optimum Injection doses and Injection Group in following experiments include of three phases (1, 2 and 3,) of animal model clinical trial which began of 1993. That’s results are very satisfactory as follows:
1. This new vaccine has been tested in many projects over safety, sterility, toxicity, endotoxin, immunogenicity and efficacy which its satisfactory results have been confirmed repeatedly.
2. The best injection group are LL (leishmaniasis without adjuvant+a reminder dose) [3, 4, 6] and the LT group (Leishmania+ Tukrium Polium as adjuvant+ a Reminder dose) [5, 7-12].
Injection doses of 100 and 200 μl/0.1 ml had the same results, which in some groups were 100 μl/0.1 ml and in some others, 200 μl/0.1 ml had better response. So we will be tested both doses again in the future.
3. New Leishmania major vaccine was prepared and accomplished its clinical trials phases (1, 2 and 3) in animal model. Its results were published in nine articles previously and finalized its results with this article after 25 years of comprehensive studies. We have obtained excellent and satisfactory results which their articles gradually have been published, and also have been presented in lectures (orals), posters and summary (abstract) forms in many internal and international congresses [3-12,26-34].
Suggestion
Our studies on the animal model and new leishmania vaccine will finished with this article. Of course, there is some other unpublished available information about clinical trials phases (1, 2 and 3) in the animal model of this new vaccine. But, we didn’t use of any other data because of this article will be larger and also, because of the lack of sufficient conditions and opportunity for publish of any other articles, we will not be able to publish any other articles related to. So far we think enough information has been provided to confirm clinical trials phases (1, 2 and 3) in the animal model of this new Leishmania vaccine. But of course, because we observed in another study on leishmania patients in different stages of the disease and people who have recovered that there is a relationship between pro-inflammatory cytokines and antioxidant and the course of the disease and the recovery of it therefore, we suggest that in the study of the vaccine on humans, pro-inflammatory cytokines, antioxidants, and neutrophil activity must also be evaluated. And concluded that it is safe and ready for testing for evaluation of clinical trial phase 1 in human model (pilot study) [35-37].
New Leishmania major vaccine was Prepared and accomplished its clinical trials phases (1, 2 and 3) in animal model. It should be noted that we used isolated amastigote from the wound for challenge for first time. 100% of the mice survived for up to 5 weeks and if further study continued, some group’s mice still survived. The results of injection doses (100 and 200 μg/0.1 ml) were almost the same, therefore we have obtained excellent and satisfactory results which show that even if this vaccine is injected and then the amastigote enters the blood of an animal model (Balb/C mice) from the wound, these mice can not only resist and have a high survival ability, but their immunological results are also very satisfactory and we were also able to determine the best dose and injection group. And the important thing in this research is that re-exposure to Leishmania amstigote has not been done in any research before.
This research has been supported by Tehran University of Medical Sciences and health Services grant 14047-30-02-90 (19/5/2011) and 15886-30-02-91(19/05/2012).
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Latifynia A, Nicknam MH, Gharagozlou MJ, Bonab SF, Ansaripour B, Charedar S, et al. (2023) Measurement of Post Challenging Immune Response (Serum’s Th1, Th2 Cytokines and Spleen’s CD Markers) with L.Major Amastigote in Balb/c Mice. 14:681.
Received: 14-Jan-2023, Manuscript No. JCCI-23-21398; Editor assigned: 17-Jan-2023, Pre QC No. JCCI-23-21398 (PQ); Reviewed: 01-Feb-2023, QC No. JCCI-23-21398; Revised: 09-Feb-2023, Manuscript No. JCCI-23-21398 (R); Published: 16-Feb-2023 , DOI: 10.35248/2155-9899.23.14.681
Copyright: © 2023 Latifynia A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.