Journal of Infectious Diseases & Preventive Medicine
Open Access
ISSN: 2329-8731
ISSN: 2329-8731
Research Article - (2022)Volume 10, Issue 3
Background: Nitazoxanide (NTZ), a broad-spectrum antiviral undergoing clinical development for treating influenza, COVID-19 and other viral respiratory illnesses, is a mild uncoupler of oxidative phosphorylation.
Methods: We evaluated the effect of Tizoxanide (TIZ), the active circulating metabolite of NTZ, on Adenosine Triphosphate (ATP) and cell viability in Madin-Darby Canine Kidney (MDCK) cells and in MDCK cells infected with influenza A and B viruses.
Results: TIZ decreased cellular ATP in a dose-dependent manner in MDCK cells and in MDCK cells infected with influenza A and B viruses. Maximum inhibition of ATP in influenza-infected or uninfected MDCK cells reached up to 45% after 6 and 24 hours of exposure to 100 μM TIZ. The decrease in cellular ATP did not affect cell viability and was reversible after eliminating TIZ from the culture. TIZ concentrations required to decrease cellular ATP levels were similar to those reported to inhibit replication of influenza A and B viruses.
Conclusion: The inhibitory effect of NTZ on replication of influenza and other respiratory viruses is related to its effect on host cell energy metabolism and downstream effects on cell signaling pathways. Modest and transient reductions of ATP do not affect viability of host cells but deny viruses’ access to energy and cellular machinery required for efficient replication.
Nitazoxanide; Antiviral; Influenza; COVID-19; Host-directed therapy; Broad-spectrum; ATP
Nitazoxanide (NTZ), a broad-spectrum antiviral agent undergoing clinical development for treating influenza, COVID-19 and other viral respiratory illnesses, is a mild uncoupler of oxidative phosphorylation [1-4]. Mechanistic studies have shown that NTZ and its active circulating metabolite, tizoxanide (TIZ), block maturation of the hemagglutinin glycoprotein of influenza viruses, the F-glycoprotein of respiratory syncytial viruses and parainfluenza viruses and the spike glycoprotein of SARS-CoV-2 [5-7]. The broad- spectrum antiviral activity of NTZ and the inability to select for resistance suggest a host target, but a specific target has not yet been clearly identified [8]. To gain further insight into the mechanism of antiviral activity of TIZ, we evaluated the effect of TIZ on ATP in MDCK cells and in MDCK cells infected with influenza A and B viruses.
Materials
Compounds: TIZ was provided by Romark (Tampa, Florida USA). Working stocks of TIZ dissolved in DMSO were prepared and stored at -80°C until used.
Cell cultures: Madin-Darby Canine Kidney (MDCK) cells were passaged in flasks and seeded in 96-well plates. Before inoculation, cells were cultured in MDCK medium and after inoculation in infection medium containing N-tosyl-L-Phenylalanyl Chloromethyl Ketone (TPCK)-treated trypsin (3 μg/mL; Sigma, T1426).
Methods
Measurement of ATP: ATP measurements were performed using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, Wisconsin USA) according to the manufacturer’s instructions, with the exception that cell culture medium was aspirated prior to adding 100 μL of ATP reagent per well. Opaque walled 96-well plates were used.
Measurement of dehydrogenase activity: Dehydrogenase activity was measured using the Cell Counting Kit-8 (Sigma-Aldrich). Twenty-μL WST-8 reagent was added to each well of 96-well plates already containing 200 μL cell culture medium per well and incubated for 1-2 hours at 37°C. After incubation, absorbance was measured at 450 nm.
Experiments in uninfected MDCK cells: Multiple identical plates with MDCK cells were prepared. Cellular ATP levels and dehydrogenase activity were measured after 6 and 24 hours of exposure to various TIZ concentrations (0 to 100 μM). Plasma concentrations of up to 30 μM to 40 μM are relevant for human exposure. Nuclei of MDCK cells were stained with DAPI (Life technologies) after treatment with TIZ for 6 or 24 hours. Images were taken using a CTL ImmunoSpot S6 Analyzer and in a representative area of the well, the cell nuclei were quantified using Immunospot software. Using identical replicate plates, the compound was removed; cells were incubated again in fresh cell culture medium (without TIZ) for 24 hours after which cell nuclei were quantified again.
Experiments in influenza virus-infected MDCK cells: Multiple identical 96-well plates with MDCK cells were (mock) infected with two influenza A and two influenza B strains for one hour at low and high MOI. After this period, the inoculum was aspirated and replaced by medium containing various TIZ concentrations (0, 10, 30, 100 μM). The cell cultures were incubated for an additional five hours (single cycle infection, high MOI) or 23 hours (multi- cycle infection, low MOI) after which cellular ATP levels were determined. In addition, cells were immunostained for influenza (for influenza A: HB65, EVL; for influenza B: MAB8661, Millipore). Images were taken using a CTL ImmunoSpot S6 Analyzer and the percentage Well Area Covered (WAC) by influenza-positive cells was quantified using Immunospot software. The virus input was checked by back titration.
Uninfected MDCK cells
Figure 1 presents cellular ATP levels and dehydrogenase activity for uninfected MDCK cells after 6 hours (A) and after 24 hours (B) exposure to TIZ at concentrations ranging from 0 μM to 100 μM. At six hours post-TIZ treatment, a concentration-dependent decrease of cellular ATP levels was observed, starting at 12.5 μM TIZ and higher with a maximum ATP decrease of 45% at 100 μM TIZ (Figure 1A). After 24 hours of treatment with TIZ, the maximum reduction was similar (44% at 100 μM); however, reduction of ATP was observed at lower concentrations of TIZ (6.25 μM) as compared to 6 hours (Figure 1B).
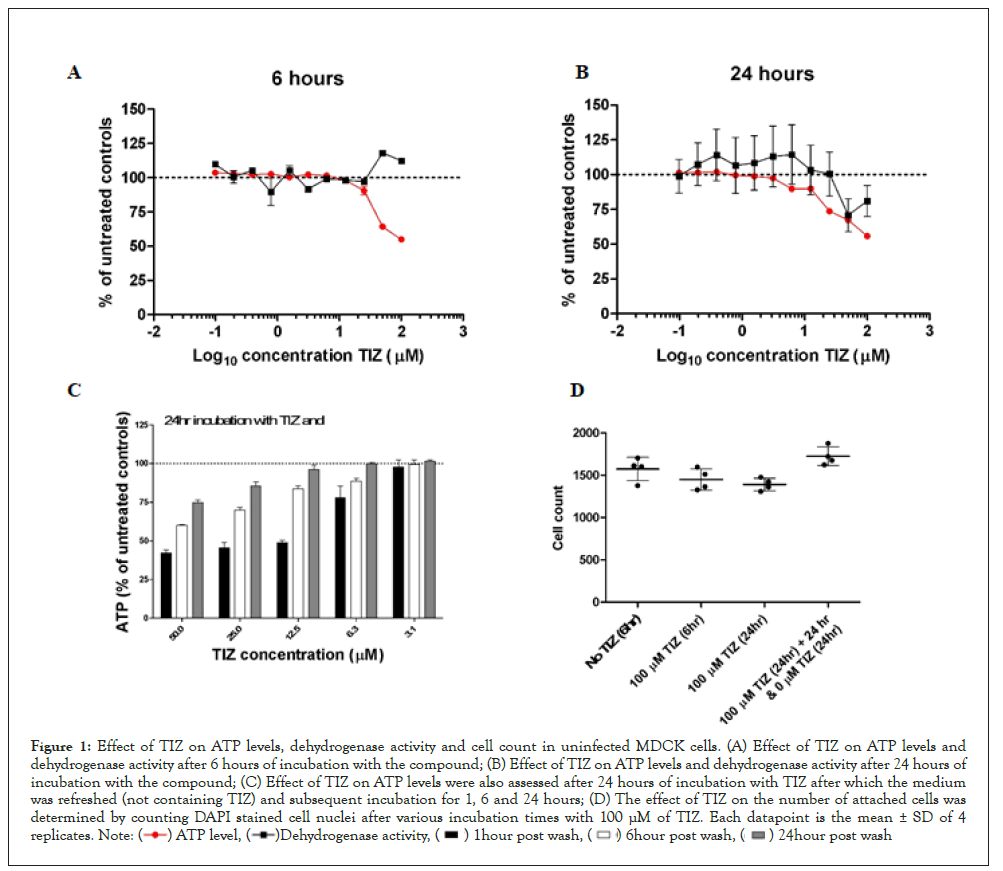
Figure 1: Effect of TIZ on ATP levels, dehydrogenase activity and cell count in uninfected MDCK cells. (A) Effect of TIZ on ATP levels and
dehydrogenase activity after 6 hours of incubation with the compound; (B) Effect of TIZ on ATP levels and dehydrogenase activity after 24 hours of
incubation with the compound; (C) Effect of TIZ on ATP levels were also assessed after 24 hours of incubation with TIZ after which the medium
was refreshed (not containing TIZ) and subsequent incubation for 1, 6 and 24 hours; (D) The effect of TIZ on the number of attached cells was
determined by counting DAPI stained cell nuclei after various incubation times with 100 μM of TIZ. Each datapoint is the mean ± SD of 4
replicates. 
To assess if TIZ affected cell viability, we also measured dehydrogenase activity because it is often used as an ATP-independent measure for cell viability. After 6 hours of exposure to TIZ, dehydrogenase activity was at the level of untreated control cells, although at high concentrations of TIZ (≥50 μM), the activity increased somewhat (Figure 1A). After 24 hours of TIZ treatment, the dehydrogenase activity decreased at higher (≥ 50 μM) TIZ concentrations (Figure 1B).
To evaluate whether the effect of TIZ on ATP is transient or enduring, MDCK cells were treated with various concentrations of TIZ for 24 hours. After this period, the cells were washed once with PBS to remove TIZ, and fresh cell culture medium (not containing TIZ) was added to the cells. After a subsequent incubation period of 1, 6, and 24 hours, the cellular ATP levels were determined (Figure 1C). In this experiment, cellular ATP levels recovered with increasing incubation time. At concentrations of 12.5 μM and lower, ATP levels recovered to the level of untreated control cells within 24 hours. However, at higher concentrations (≥ 25 μM), full recovery did not occur within the timeframe of 24 hours.
To exclude the possibility that reduced ATP levels and dehydrogenase activity are due to a reduced number of cells in the wells (due to detachment, apoptosis and necrosis for instance), MDCK cells nuclei were stained with DAPI and quantified once TIZ treatment was completed (Figure 1D). The number of cells did not significantly decrease after treatment with 100 μM TIZ for 6 or 24 hours.
Moreover, when cells were incubated with 100 μM TIZ, after which the compound was removed and incubated again in fresh cell culture medium for 24 hours, the number of nuclei increased, most likely due to cell proliferation.
Influenza virus-infected MDCK cells: We then measured ATP levels in MDCK cells infected with two influenza A virus strains and two influenza B virus strains using both single cycle (6 hours, multiplicity of infection (MOI) > 1) and multicycle (24 hours, MOI ≤ 0.01) infection. For both single- and multicycle infection (Figures 2A and 2B), TIZ reduced ATP in influenza-infected cells in a concentration-dependent manner, similarly to its effect in uninfected cells. For the single cycle infection (Table 1), back titration showed that the MOI used in the assay varied between 1.2 and 20.4. Virus replication was assessed by influenza-specific immunostaining of the cell monolayer. The well area covered by the immunostaining was calculated and is a measure of virus replication. The maximum reduction of well area covered by influenza virus spots was 3.7-fold when cell cultures were treated with 100 μM TIZ. At 30 and 10 μM, the reduction was at most 1.5-fold.
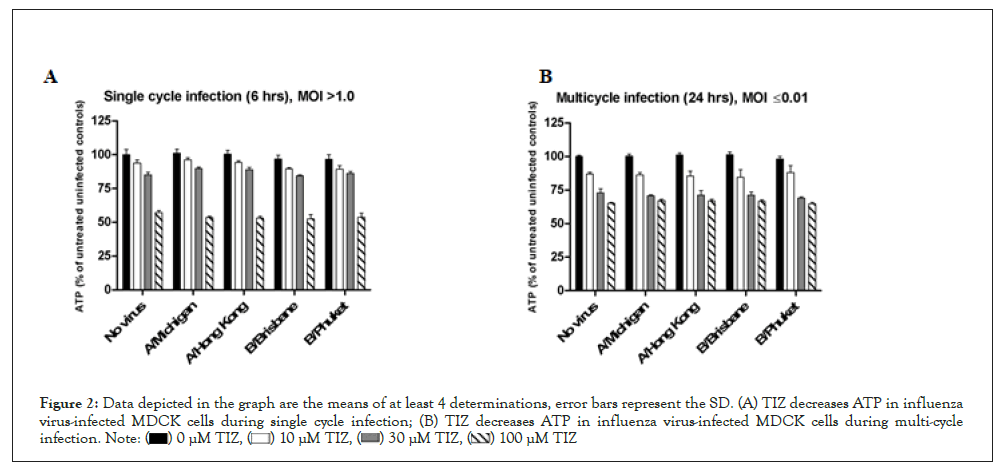
Figure 2: Data depicted in the graph are the means of at least 4 determinations, error bars represent the SD. (A) TIZ decreases ATP in influenza
virus-infected MDCK cells during single cycle infection; (B) TIZ decreases ATP in influenza virus-infected MDCK cells during multi-cycle
infection.
| Single cycle infection (6 hrs), MOI>1.0 | MOI | Well area covered by influenza virus spots (%) | Fold reduction | Well area covered by influenza virus spots (%) | Fold reduction | Well area covered by influenza virus spots (%) | Fold reduction | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 µM TIZ | 10 µM TIZ | 0 µM TIZ | 30 µM TIZ | 0 µM TIZ | 100 µM TIZ | |||||
| A/Michigan | 6.2 | 13.6 | 18.9 | 0.7 | 18.1 | 17.3 | 1 | 15.1 | 4.7 | 3.2 |
| A/Hong Kong | 1.2 | 1.6 | 1.7 | 0.9 | 4.1 | 2.8 | 1.5 | 3 | 1.2 | 2.4 |
| B/Brisbane | 9.1 | 6.2 | 8.7 | 0.7 | 10.1 | 8.9 | 1.1 | 10.6 | 3.5 | 3 |
| B/Phuket | 20.4 | 9 | 11.3 | 0.8 | 8.7 | 9.6 | 0.9 | 14.2 | 3.8 | 3.7 |
Table 1: Fold reduction in well area covered (WAC) by influenza virus spots (%) in TIZ treated cultures compared to untreated cultures in single cycle infection.
For the multicycle infection (Table 2), back titration results showed that the MOI used in the assay varied between 0.004 and 0.01. Full inhibition of virus replication was observed for B/Phuket when incubated with 100 μM TIZ. The negative Well Area Covered (WAC) for this culture condition (-0.2) is because the background WAC was slightly higher than the specific WAC of this culture condition; hence, no fold reduction could be calculated, which was denoted with “∞”. For the other viruses, treated with 100 μM TIZ, the fold reduction varied between 5.2 and 29.8-fold. At 30 and 10 μM TIZ, the reduction was at most 5.2-fold. It has to be noted that the fold reduction for A/Michigan at 100 and 30 μM TIZ is likely to be larger than could be calculated since the wells not treated with TIZ were completely positive for influenza NP, meaning that uninhibited virus propagation was capped by the well area.
| Multicycle infection (24 hrs), MOI≤1.00 | MOI | Well area covered by influenza virus spots (%) | Fold reduction | Well area covered by influenza virus spots (%) | Fold reduction | Well area covered by influenza virus spots (%) | Fold reduction | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 µM TIZ | 10 µM TIZ | 0 µM TIZ | 30 µM TIZ | 0 µM TIZ | 100 µM TIZ | |||||
| A/Michigan | 0.01 | 25 | 19.5 | 1.3 | 23 | 14.9 | 1.5 | 23 | 4.4 | 5.2 |
| A/Hong Kong | 0.01 | 13 | 12.3 | 1.1 | 13.6 | 4 | 3.4 | 20.8 | 0.7 | 29.8 |
| B/Brisbane | 0.004 | 3.5 | 1.5 | 2.4 | 3 | 0.8 | 3.7 | 4.6 | 0.3 | 18.5 |
| B/Phuket | 0.01 | 7.8 | 5.1 | 1.5 | 7.4 | 1.4 | 5.2 | 9.9 | -0.2 | ∞ |
Table 2: Fold reduction in well area covered (WAC) by influenza virus spots (%) in TIZ treated cultures compared to untreated cultures in Multicycle infection.
Percentages shown are the means of at least 4 replicates. Background %WAC (uninfected cell controls) is subtracted to calculate the specific %WAC of each culture condition, shown here.
These data show that TIZ decreases cellular ATP in a dose- dependent manner in uninfected MDCK cells and in MDCK cells infected with influenza A and B viruses. Maximum inhibition of ATP in influenza infected or uninfected MDCK cells reached up to 45% after 6 and 24 hours of exposure to 100 μM TIZ. The decrease in cellular ATP did not affect cell viability and was reversible after removing TIZ from the culture. Concentrations of TIZ required to decrease cellular ATP levels (6.25 to 12.5 μM) were similar to those reported to inhibit replication of influenza A and B viruses in cell culture assays. Plasma concentrations up to approximately 30 μM to 40 μM are relevant for human exposure.
Studies of a number of different viruses have shown that viral replication is ATP-dependent Braakman et al. first described the role of ATP in the formation of disulphide bonds during the maturation and the protein folding of the influenza hemagglutinin in the endoplasmic reticulum [9,10]. Others have shown that ATP regulates the assembly and transport of vesicular stomatitis virus G protein trimers and that increased ATP generation in host cells is required for efficient vaccinia virus production [11,12]. Similarly, Mirazimi and Svensson reported that ATP is essential for correct folding and disulphide bond formation in rotavirus [13].
Mechanistic studies in virus-infected cell lines have shown that treatment with NTZ or TIZ inhibits maturation of the hemagglutinin glycoprotein of influenza viruses, the F-glycoprotein of respiratory syncytial viruses and parainfluenza viruses and the spike glycoprotein of SARS-CoV-2 [5-7]. Inhibition of ATP and the consequential activation of the AMP-activated protein kinase (AMPK) have also been associated with suppression of secretion of pro-inflammatory cytokines important in viral respiratory illnesses [14-16]. Notably, we have observed in cell bioassays that TIZ inhibits secretion of TNF-alpha, Il-2, IL-4, IL-5, IL-6, IL-8 and IL- 10 with IC50s ranging from 0.67 μg/mL to 2.65 μg/mL. Hong et al studied the potential for NTZ to inhibit IL-6 in vitro and in vivo. In those studies, NTZ inhibited lipopolysaccharide (LPS)-induced production of IL-6 from RAW 264.7 cells and mouse peritoneal macrophages with 50% inhibitory concentrations (IC50s) of 1.54 μM and 0.17 μM, respectively [17]. NTZ also inhibited the LPS- induced expression of IL-6 mRNA in RAW 264.7 cells. In vivo, NTZ was administered orally at a dose of 100 mg/kg to mice 2 hours before a 1 mL intraperitoneal injection of 4% Thioglycollate (TG). Six hours after TG injection, plasma IL-6 levels were markedly lower (by 90%) than the levels in vehicle-treated mice [17].
The inhibitory effect of NTZ on replication of influenza and other respiratory viruses is related to its effect on host cell energy metabolism and downstream effects on cell signaling pathways. Modest and transient reductions of ATP do not affect viability of host cells but deny viruses’ access to energy and cellular machinery required for efficient replication. The effect on host cell energy metabolism and consequential activation of AMPK could also play a role in inhibiting secretion of pro-inflammatory cytokines, which are relevant for viral respiratory illnesses.
JFR is an employee of and owns an equity interest in Romark Laboratories, L.C.
Citation: Rossignol JF, Tijsma ASL, Baalen CAV (2022) Mechanism of Antiviral Activity of Nitazoxanide: Effect on Adenosine Triphosphate in Influenza-Virus Infected Madin Darby Canine Kidney Cells. J Infect Dis Preve Med. 10:262.
Received: 21-Mar-2022, Manuscript No. JADPR-22-16320; Editor assigned: 24-Mar-2022, Pre QC No. JADPR-22-16320 (PQ); Reviewed: 13-Apr-2022, QC No. JADPR-22-16320; Revised: 20-Apr-2022, Manuscript No. JADPR-22-16320 (R); Published: 27-Apr-2022 , DOI: 10.35841/2329-8731.22.10.262
Copyright: © 2022 Rossignol JF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.