Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Original Research Article - (2019)Volume 15, Issue 1
Mechanism of Low Endotoxin Recovery (LER) was investigated by observing the change in particle sizes and activity of endotoxin in LER solutions containing sodium citrate and polysorbate 20. Interestingly, endotoxin aggregates were not dispersed to be smaller particles under LER conditions according to the decease of the activity. This observation was different from previous predictions of the mechanism. Endotoxin aggregates were observed by Dynamic Light Scattering, and activity was measured by the Limulus amebocyte lysate test. Particles with sizes similar to endotoxin aggregates were always observed despite different remaining activity of spiked endotoxin. This observation differed from previously proposed mechanisms for LER that dispersed endotoxin aggregates were smaller in sizes. Based on the findings, a new mechanism of LER is proposed. Purified endotoxin forms non-lamellar cubic structures in water, and the endotoxin aggregates are reinforced by divalent cations. When purified endotoxin is added to LER solutions, divalent cations are removed from outer layer of endotoxin by the chelating agent, and the loss of divalent cations weakens the outside layer of the endotoxin aggregates. The endotoxin aggregation structure is maintained at low temperature, but endotoxin molecules on the surface of the endotoxin aggregates are gradually replaced with detergent molecules at higher temperature. This replacement reduces the surface area of the endotoxin, and cause reduction of activity of the endotoxin aggregates. The apparent sizes of the aggregates are not changed after the replacement.
Low endotoxin recovery; Endotoxin; Limulus amebocyte lysate; Lipopolysaccharide; Bacterial endotoxins test
Endotoxin (lipopolysaccharide, LPS) is a component of the outer membrane of Gram-negative bacteria, and demonstrates multiple biological activities, such as pyrogenicity [1]. Parenteral drugs and medical devices are required to be tested by the Bacterial Endotoxins Test (BET) using Limulus amebocyte lysate (LAL) to avoid releasing products contaminated with endotoxin because of its high biological activity [2]. Low Endotoxin Recovery (LER) has been one of the most controversial recent topics in the BET for biopharmaceuticals. LER is a phenomenon of a loss of spiked endotoxin activity in a neat sample, and is observed with samples containing a chelating agent and a detergent [3]. U.S. Food and Drug Administration (FDA) recommended hold-time studies to establish procedures for storing and handling of products [4]. LER was observed during the hold-time studies for the biopharmaceutical products, and the lost endotoxin activity was not recovered by simple water dilution, the most popular method to overcome interference of samples. The BET requires the interfering factors test to avoid inhibition or enhancement of the LAL reaction by a sample to be tested [2]. The interfering factors test is based on the confirmation of acceptable recovery (50%-200%) in a diluted sample. Samples in LER studies were usually diluted with water for the BET, and the diluted samples were confirmed to overcome interference of the sample by the interfering factors test [5-10]. This indicated that the diluted samples were not inhibitory to the activation of LAL, and that endotoxin activity in the diluted samples should be detected by the BET if there was still activity of the spiked endotoxin. Endotoxin activity spiked in the neat samples was decreased during the storage under LER conditions.
The mechanism of LER is not clear, even though some hypotheses were proposed [7,11]. The previously proposed hypotheses are based on the monomerized LPS generated under LER conditions becoming inactive to the LAL reagents. LPS is an amphipathic substance, and forms aggregates in water [1]. The aggregation status of LPS affects its biological activities [12,13], and monomeric LPS is probably not biologically active [12,14]. Therefore, the proposed hypotheses are possible, but there are no supporting data for them.
Characteristics of LER have been reported [7,9]. LER is affected by temperature [7,9], pH [7,9], salt concentration [9], chelating agent concentration [9], detergent concentration [7,9], types of chelating agent [7,9,15], and sorts of detergent [7,15]. The reaction rate constants of Reference Standard Endotoxin (RSE) in solutions containing sodium citrate and polysorbate 20 were temperature, pH, and salt concentration dependent [9]. Considering that the conditions reported were not destructive for LPS, LPS was not decomposed under the LER conditions, and LPS molecules should be still in the solutions, but the status of the LPS was changed under LER conditions. Tsuchiya [15] demonstrated that RSE activity was maintained at 4°C for a long time in solutions containing a chelating agent and a detergent. The activity was decreased when the solutions were diluted with water, although the activity was maintained by dilution with a magnesium solution. This finding suggests that the water dilution is not suitable for samples under LER conditions, and that the mechanism of LER may be different from the proposed hypotheses.
The purpose of this study is to propose a mechanism of LER based on the data including the change in aggregation status of LPS in solutions containing a chelating agent and a detergent (LER solutions). LPS aggregates were observed in LER solutions by using dynamic light scattering (DLS), and the endotoxin activity was measured by the LAL test. If the previously proposed hypotheses are relevant, dispersion of the LPS aggregation should be observed. Surprisingly, the results revealed that particles with similar sizes to the LPS aggregates still maintained in the LER solutions after the endotoxin activity decreased. These data suggested a different mechanism for LER.
Sample preparation
Stock solutions were prepared from reagent-grade chemicals, and were filtered with positively charged 0.2 μm filters (Acrodisc® Syringe Filter 0.2 mm Posidyne® Membrane, Pall Life Science, Ann Arbor, MI, USA). Endotoxin was not detected in the stock solutions by the kinetic chromogenic LAL method (KCA). Water used was LAL Reagent Water (LRW, Charles River, Charleston, SC, USA), and 5% sodium chloride was purchased from B/Braun (5% Sodium Chloride Injection USP, Irvine, CA, USA). The USP reference standard endotoxin (RSE, 10,000 USP Endotoxin Units (EU) per vial) was reconstituted with 5 mL LRW, and diluted with LRW to preferable concentrations. For the long term observation, LPS without any additives (Endotoxin Indicator EVV100K, Charles River) was used.
Sample dilution methods and measurement of endotoxin activity
Endotoxin activity was measured by the KCA using a chromogenic LAL (Endochrome-K™, Charles River). The LAL reagents were reconstituted with the endotoxin-specific buffer (BG120, Charles River) to eliminate possible glucan interference. Endotoxin standard curves were established by logarithmic plotting of endotoxin concentrations versus onset times (reaction time to obtain 5% decrease of transmittance of a reaction mixture). Quadratic regression was used to calculate endotoxin concentrations in samples.
Samples were diluted by three dilution methods, the direct assay method (direct method), the water dilution method (water dilution), and the magnesium dilution method (magnesium dilution), as previously described (15). Briefly, for the direct method, a 0.01 mL of a sample was added to a glass reaction tube containing 0.1 mL LAL and 0.1 mL LRW, and the reaction mixture was set on a tube reader (Toxinometer™, Wako Pure Chemical Industries, Ltd., Osaka, Japan) to perform the KCA. For the water dilution, samples were diluted with LAL Reagent Water (Charles River) in polystyrene test tubes (Falcon 352054, BD Biosciences, Bedford, MA, USA), and the diluted samples were measured by the KCA using 1:1 ratio of the LAL reagent and the samples. For the magnesium dilution, samples were diluted with 2 mM magnesium solution (250-fold dilution of Cation Tris buffer (BC1000, Charles River)) in polypropylene test tubes. Diluted samples were measured by the KCA as same as the water dilution.
Observation of particles in samples by dynamic light scattering (DLS)
Particle sizes distributions were measured by DLS on a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern Worcs, UK) with a scattering angle at 173°. Distribution of averaged intensity of 3 to 6 measurements against particle sizes was drawn on a bar chart for each sample, and divided areas were measured for the particle sizes at <3 nm, 3-10 nm, 10-30 nm, 30-100 nm, 100-300 nm, 300-1000 nm, 1000-3000 nm, and >3000 nm. The ratio for each range of the particle sizes was calculated by dividing the area of each range by the total area. Peaks of intensity on each chart were ranked from the highest one, and the particle sizes for the peaks were recorded.
Intensity distribution of LER solution, RSE solution, and the mixture
Figure 1a shows the intensity distribution of RSE dilutions at 200 EU/mL and LER solutions containing 10 mM sodium citrate and 0.05% polysorbate 20 on DLS.
Table 1 shows the intensity ratios and the peaks for each sample. For RSE solutions, most of the particles were observed in the range of 100-1000 nm, and the peak was at 300-450 nm. On the other hand, the LER solutions showed a peak at 10 nm that is considered as micelles of polysorbate 20.
| Sample | Intensity | Peak (nm) | |||
|---|---|---|---|---|---|
| 3-30 nm | 100-1000 nm | 1st | 2nd | 3rd | |
| 10 mM Sodium citrate | 84% | 11% | 10 | 712 | none |
| 0.05% Polysorbate 20 | 92% | 0% | 10 | 5560 | 0.83 |
| RSE 200 EU/mL | 0% | 100% | 342 | none | none |
| 0% | 85% | 428 | 5560 | none | |
| 0% | 88% | 342 | 5560 | 79 | |
| 0% | 94% | 396 | 5560 | none | |
Table 1: Intensity distribution and peaks of an LER solution and an RSE dilution.
These results indicated that RSE at 200 EU/mL and the LER solution were represented by the intensity in the particle sizes at 100-1000 nm and 3-30 nm, respectively. Figure 1b shows the intensity distribution of 200 EU/mL RSE in an LER solution containing 10 mM sodium citrate, 0.05% polysorbate 20, and 0.8% sodium chloride. Table 2 shows the intensity ratio, peaks of the particle sizes, and endotoxin activity with the direct method, the water method, and the magnesium method in the LER solutions containing 200 EU/mL RSE on day 0 and day 7 at 23°C. Because both peaks at 3-30 nm and 100-1000 nm were observed in the mixture on day 0, DLS was considered to be useful for the observation of RSE aggregates in LER solutions.
RSE activity was decreased to less than 0.5% in the LER solution at 23°C for 7 days (Table 2). Despite the difference of the RSE activity, intensity distribution on day 7 was similar to that on day 0.
| Storage period (days) | Intensity | Peak (nm) | Endotoxin (EU/mL) (%Recovery) | |||||
|---|---|---|---|---|---|---|---|---|
| 3-30 nm | 100-1000 nm | 1st | 2nd | 3rd | DM | WD | MD | |
| 0 | 30% | 67% | 396 | 8.7 | 5560 | 351 (176%) | 195 (97%) | 286 (143%) |
| 7 | 34% | 62% | 423 | 8.7 | 5560 | 0.4 (0.20%) | 0.14 (0.068%) | 0.26 (0.13%) |
LER solution: 10 mM sodium citrate, 0.05% polysorbate 20, 0.8% sodium chloride, and 200 EU/mL RSE. DM: Direct method; WD: Water dilution; MD: Magnesium dilution.
Table 2: Effect of storage at 23°C for 7 days on intensity distribution, peaks, and endotoxin activity in an LER solution containing RSE.
Characteristics of diluted LER solution containing RSE
Similar intensity distributions were observed in the dilutions of LER solutions containing RSE by the water dilution and the magnesium dilution (Figure 2). There was no significant difference in intensity distribution between these dilutions of the LER solution containing RSE stored at 4°C for 7 days, despite the endotoxin activity in the magnesium dilution being 214 times higher than that in the water dilution when stored at 4°C (Table 3).
| Dilution method | Storage | Endotoxin (EU/mL) (%recovery) | Intensity | Peak (nm) | ||||
|---|---|---|---|---|---|---|---|---|
| 3-30 nm | 100-1000 nm | 1st | 2nd | 3rd | 4th | |||
| WD | 4°C for 7 days | 0.14 (0.71%) | 21% | 60% | 295 | 5560 | 10 | 0.96 |
| MD | 29.9 (150%) | 20% | 64% | 342 | 5560 | 10 | 1.1 | |
| WD | 23°C for 7 days | 0.014 (0.068%) | 21% | 57% | 295 | 5560 | 10 | 0.62 |
| MD | 0.026 (0.13%) | 24% | 46% | 5560 | 295 | 12 | 0.96 | |
LER solution: 10 mM sodium citrate, 0.05% polysorbate 20, 0.8% sodium chloride, and 200 EU/mL RSE. DM: Direct Method; WD: Water Dilution; MD: Magnesium Dilution.
Table 3: Comparison of endotoxin activity, intensity, and peaks of LER solution containing RSE between storages at 4°C and 23°C.
The LER solution containing RSE stored at 23°C for 7 days showed less than 0.2% endotoxin activity and major intensity distribution was observed in the range of 100-1000 nm (Table 3). A peak at around 300 nm was observed for all the samples, but the intensity distribution was broader in those dilutions (Figure 2) than undiluted LER solutions (Figure 1b).
Long term observation of intensity distribution of LER solutions containing LPS
LPS without any additives (100,000 EU/vial) was dissolved with 5 mL of an LER solution containing 10 mM sodium citrate and 0.05% polysorbate 20. Intensity distribution was measured on day 0, day 1, day 372, day 390, and day 872 (Figure 3). Table 4 shows the summary of the results. The peak at around 300 nm (295-369 nm) was maintained for 872 days, but the peak at around 9 nm was gradually decreased. The endotoxin activity of the solution was measured on day 377 and day 872. The activity detected with the direct method and the water dilution was less than 0.003% on both days. The activity with the magnesium dilution was higher than that with the direct method and the water dilution, but all were less than 0.5% of the original activity.
| Storage Period (days) | Intensity | Peak (nm) | Endotoxin (EU/mL) (%recovery) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3-30 nm | 100-1000 nm | 1st | 2nd | 3rd | 4th | DM | WD | MD | |
| 0 | 35% | 61% | 342 | 8.7 | 106 | nd | nd | nd | |
| 1 | 35% | 61% | 342 | 8.7 | 5560 | 68 | nd | nd | nd |
| 372 | 14% | 73% | 295 | 8.7 | 79 | 5560 | nd | nd | nd |
| 377 | nd | nd | nd | nd | nd | 0.036 (0.00018%) | 0.47 (0.0023%) | 63.9 (0.32%) | |
| 390 | 9.00% | 76% | 369 | 5560 | 8.7 | nd | nd | nd | |
| 872 | 0.00% | 80% | 342 | 141 | 5560 | 0.57 (0.0028%) | 0.44 (0.0022%) | 85.1 (0.43%) | |
LER solution: 10 mM sodium citrate, 0.05% polysorbate 20, 0.8% sodium chloride, and 200 EU/mL RSE. DM: Direct method; WD: Water dilution; MD: Magnesium dilution. nd: not determined.
Table 4: Long term observation of intensity distribution, peaks, and endotoxin activity in LER solution containing purified LPS.
The mechanism of the typical LER caused by a chelating agent and a detergent is not clearly elucidated, but there were some hypotheses proposed. Tsuchiya analyzed the previous literature, and considered that LER is caused by a change in LPS aggregation to smaller sizes [11]. Reich et al. proposed a two-step mechanism for the typical LER; pure LPS aggregates are altered by chelators increasing the permeability of the aggregates, then detergents interact with LPS by forming mixed aggregates containing monomerized LPS [7]. Both hypotheses predicted that LPS large aggregates were converted to small mixed aggregates. However, there was no evidence to show decrease of the large LPS aggregates or increase of the small mixed aggregates.
This study aims to observe change in aggregation status of LPS under LER conditions. Dynamic light scattering (DLS) was applied to detect the large LPS aggregates and the small detergent micelles. Fortunately, LPS aggregates showed an intensity peak in the range between 100 nm and 1000 nm that was different from that of polysorbate 20 at around 10 nm (Figure 1a and Table 1), and both intensity peaks were observed in the LER solution containing RSE (Figure 1b and Table 2). In other words, LPS aggregates were discriminated from polysorbate 20 micelles by the intensity distribution on DLS. Even though DLS may not be suitable for the morphological observation of LPS aggregates [16], it was still useful for observation of the change of aggregation and micelle sizes in LER solutions.
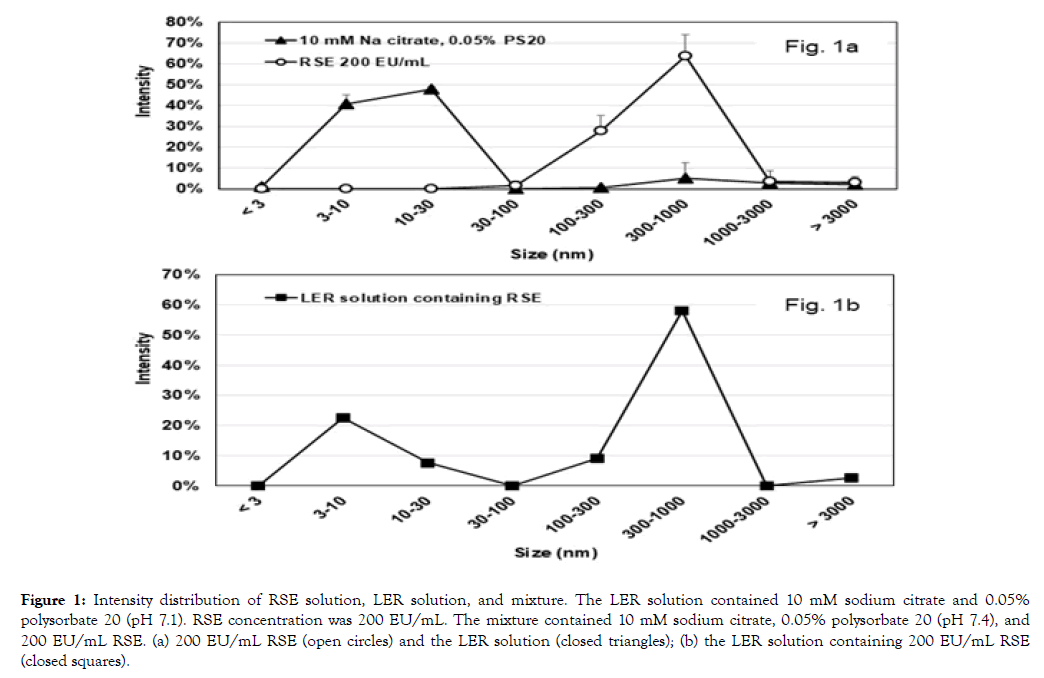
Figure 1. Intensity distribution of RSE solution, LER solution, and mixture. The LER solution contained 10 mM sodium citrate and 0.05% polysorbate 20 (pH 7.1). RSE concentration was 200 EU/mL. The mixture contained 10 mM sodium citrate, 0.05% polysorbate 20 (pH 7.4), and 200 EU/mL RSE. (a) 200 EU/mL RSE (open circles) and the LER solution (closed triangles); (b) the LER solution containing 200 EU/mL RSE (closed squares).
If the mechanism of LER is reduction of the sizes of LPS aggregates, the particle sizes of LPS aggregates should be gradually getting smaller. According to the results in this study, however, particles were always observed at sizes consistent with LPS aggregates regardless of activity;
(1) RSE activity was decreased to less than 0.5% in an LER solution at 23°C for 7 days (Table 2). The RSE representative peak was observed at around 400 nm on both day 0 and day 7, and the intensity distribution in the range of 100-1000 nm was similar (Table 2).
(2) The magnesium dilution and the water dilution recovered 150% and 0.71% RSE activity in an LER solution stored at 4°C for 7 days, respectively (Table 3). Despite the difference in activity, the intensity distribution and the peaks of the intensity were similar between the dilutions in these methods (Figure 2 and Table 3).
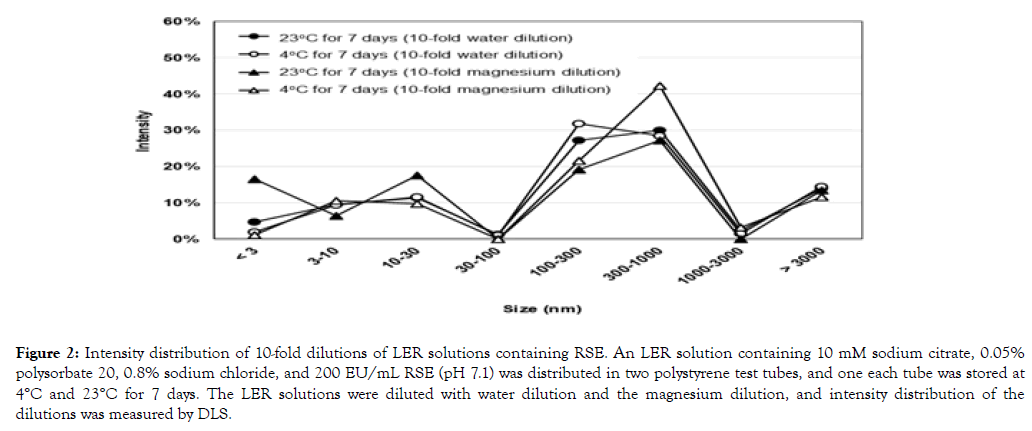
Figure 2. Intensity distribution of 10-fold dilutions of LER solutions containing RSE. An LER solution containing 10 mM sodium citrate, 0.05% polysorbate 20, 0.8% sodium chloride, and 200 EU/mL RSE (pH 7.1) was distributed in two polystyrene test tubes, and one each tube was stored at 4°C and 23°C for 7 days. The LER solutions were diluted with water dilution and the magnesium dilution, and intensity distribution of the dilutions was measured by DLS.
(3) The peak of LPS aggregates at around 300 nm was continuously observed in the LER solution containing high concentration of purified LPS for 872 days at 23°C, even though the activity was decreased to less than 0.5% with all dilution methods (Figure 3 and Table 4).
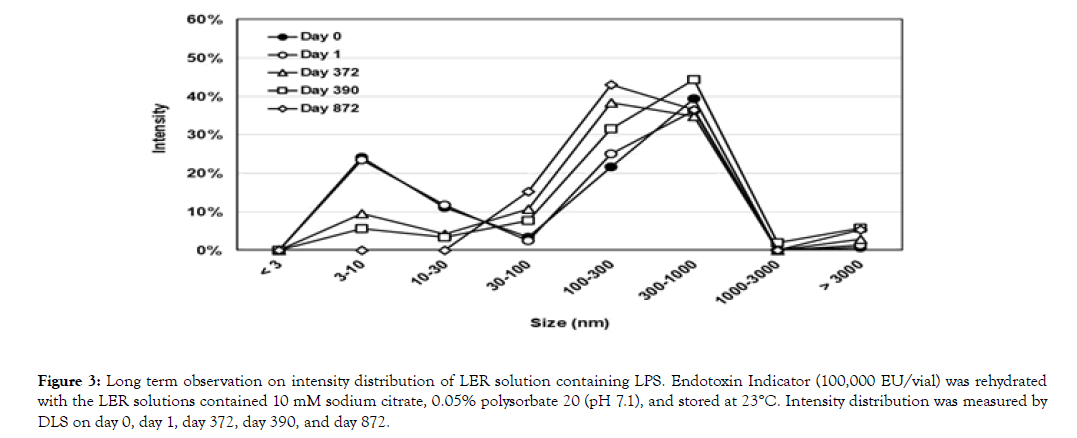
Figure 3. Long term observation on intensity distribution of LER solution containing LPS. Endotoxin Indicator (100,000 EU/vial) was rehydrated with the LER solutions contained 10 mM sodium citrate, 0.05% polysorbate 20 (pH 7.1), and stored at 23°C. Intensity distribution was measured by DLS on day 0, day 1, day 372, day 390, and day 872.
(4) The peak of detergents at around 10 nm was gradually decreased in the LER solutions stored at 23°C (Figure 3 and Table 4). After 872 days storage, the peak at around 10 nm was not observed in the LER solution. This observation suggests that the equilibrium status of the particles in the LER solution is not a solution with small particles of mixed micelles of the detergent and monomeric LPS. In other words, terminal forms of LPS in LER solutions are not small mixed aggregates that are previously predicted [7,11].
All observations in this study indicated that dispersion of large LPS aggregates was not the major cause of LER.
LAL activation is triggered by intermolecular activation of Factor C molecules on LPS aggregates [17]. The Factor C activation occurs on the surface of LPS aggregates after active transition state Factor C forms the dimer or its multimers on the LPS aggregates. This suggests that LPS activity depends on the surface area of LPS aggregates. Therefore, the decrease of LPS activity in LER solutions is caused by reduction of available surface area of LPS aggregates. This Factor C activation system also suggests that a certain space of LPS aggregates is necessary for the Factor C activation. In other words, monomeric LPS cannot activate Factor C. This supports the results of Mueller et al. [14] that aggregates are the biological units of endotoxin. The mixed aggregates of detergents containing monomeric LPS can be generated in LER, and are probably inactive to the LAL, no matter whether they are the major cause of LER or not.
The RSE activity was maintained in the LER solutions at 4°C for at least 38 days, and decreased quickly after being diluted with water [15]. This reaction seemed to be temperature dependent because the recovery of endotoxin was affected by the water temperature for the dilution. For example, using an LER solution containing 20 EU/mL RSE stored at 4°C for 13 days, RSE activity was recovered 70% after the dilution with water at 5°C regardless of 9.2% recovery with water at 25°C (data not shown). The activity change in water dilution is quicker than that in LER, and may be caused by the condition change of weakened LPS aggregates by dilution. The magnesium dilution can reinforce the weaken LPS aggregates in LER solution to prevent this change.
LER was found in the BET using RSE or Control Standard Endotoxin (CSE) that are prepared from purified LPS. Purified LPS forms a non-lamellar cubic structure at a temperature below the phase transition temperature between a highly ordered gel or β-phase and a disordered liquid-crystalline or α-phase [13]. The transition temperature for S-form LPS is usually higher than 24°C [18]. Therefore, RSE or CSE probably forms a nonlamellar cubic structure in solutions at room temperature, and may be rigid at a low temperature. This can be one of the reasons why endotoxin activity is maintained in LER solutions at a low temperature.
It was reported that considerable amounts of magnesium and calcium were observed even after electrodialysis [19]. This suggests that divalent cations are difficult to remove from the inside of the LPS aggregates. The chelating agent can remove divalent cations from the surface of the LPS aggregates in LER solutions, but may not be able to access the inner layers of the LPS aggregates. Therefore, there is a possibility that LER reaction occurs only on the surface of LPS aggregates.
Based on the above findings in this study, the mechanism of LER should satisfy following conditions; (1) apparent LPS aggregation sizes are not changed regardless of the activity, (2) the LPS aggregates are maintained in LER solutions at a low temperature, and (3) its activity is rapidly decreased when it is diluted with water. A following mechanism is proposed to satisfy the conditions. The first step of LER is removal of divalent cations from the surface of LPS aggregates that form a nonlamellar cubic structure, and the surface LPS layers are weakened. Then the LPS molecules on the surface of the LPS aggregates can be replaced with detergent molecules, but this is temperature dependent. The weakened LPS surface is rigid at a low temperature, and the LPS aggregates are maintained until the condition is changed. The replacement of the LPS molecules with detergent molecules gradually proceeds at a higher temperature, such as room temperature. Then the surface area of LPS on the LPS aggregates is decreasing according to the replacement, and this causes LPS activity reduction. This can proceed more rapidly when the condition is changed. Dilution with water is one of the condition changes. Figure 4 illustrates the proposed mechanism of LER in this study.
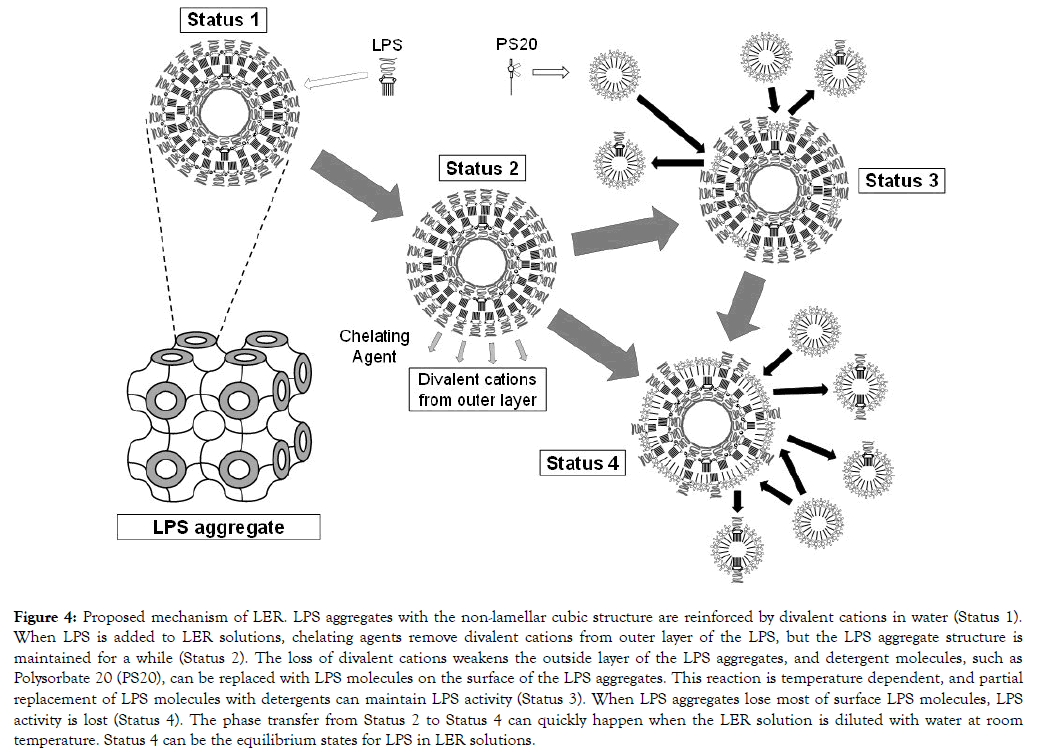
Figure 4. Proposed mechanism of LER. LPS aggregates with the non-lamellar cubic structure are reinforced by divalent cations in water (Status 1). When LPS is added to LER solutions, chelating agents remove divalent cations from outer layer of the LPS, but the LPS aggregate structure is maintained for a while (Status 2). The loss of divalent cations weakens the outside layer of the LPS aggregates, and detergent molecules, such as Polysorbate 20 (PS20), can be replaced with LPS molecules on the surface of the LPS aggregates. This reaction is temperature dependent, and partial replacement of LPS molecules with detergents can maintain LPS activity (Status 3). When LPS aggregates lose most of surface LPS molecules, LPS activity is lost (Status 4). The phase transfer from Status 2 to Status 4 can quickly happen when the LER solution is diluted with water at room temperature. Status 4 can be the equilibrium states for LPS in LER solutions.
LER is activity change of LPS, and not interference with the LAL reaction. The activity change of LPS is related to LPS states in the solution. Numerous studies have been reported on the relationship between LPS states and biological activities, and LPS activity changes caused by coexisting substances were also reported. Examples are listed in Table 5. There is still a controversial discussion whether monomers or aggregates are the biologically active units [12,14,20,21]. Some of them measured the activity with the LAL test, and showed the correlation between the LAL test and the cell activations [14,21]. Takayama et al. [21] observed LAL activation with their monomeric LPS preparations, and Mueller et al. [14] did not. As discussed above, a recent study revealed that monomeric LPS cannot activate Factor C [17]. Although there was a proposed mechanism that Factor C can be activated by monomeric LPS before [22], no direct evidence was provided. Therefore, there is a possibility that the Takayama’s monomeric LPS preparations were partially reaggregated and that the regenerated LPS aggregates reacted with the LAL. Certain metal ions decreased LPS activity [23-27]. Fujita et al [26] observed change in LPS aggregates by gel-filtration, and LPS solution with iron or cupper did not show the peaks of the original LPS solution probably because of increased particle sizes of LPS. This suggests that larger LPS aggregates show lower LAL activation than smaller aggregates. There are some studies to show decrease of LPS aggregate sizes [28-30]. The decrease of LPS aggregate sizes provided higher biological activities. In summary, smaller LPS aggregates have higher activity to LAL than larger ones, and monomeric LPS is not active to LAL. The controversial discussion on other biological activities is not discussed in this study because more studies may be necessary to conclude it. The proposed LER mechanism involves some assumptions; dispersed mixed micelles of monomeric LPS and detergents are not active to the LAL, and the activity of LPS depends on the surface area of its aggregates. Above considerations support these assumptions.
| Reference | Methods | Treatment | LPS states | Remarks |
|---|---|---|---|---|
| Monomeric LPS is not biologically active | ||||
| Ribi et al. [12] | Pyrogen test | Deoxycholate | Monomeric/Aggregates | No pyrogenicity with monomeric LPS. |
| Mueller et al. [14] | LAL test, cytokine production | Dialysis | Monomeric/Aggregates | Monomeric LPS/Lipid A were not active. |
| Monomeric LPS is biologically active | ||||
| Takayama et al. [20] | B cell activation | Dialysis, BSA* added | Monomeric/Aggregates | Both monomeric and aggregated LPS were active. |
| Takayama et al. [21] | LAL test, macrophage response | Dialysis, BSA* added | Monomeric/Aggregates | Monomeric LPS showed higher activity. |
| Samller sizes of LPS aggregates are more active than larger ones | ||||
| Galanos et al. [28] | Anti-complementary activity | Electrodialysis | Different sizes among salt forms | High activity with smaller LPS aggregates. |
| Komuro et al. [29] | Pyrogen test, LAL test | Sonication | Fractionated | Optimum sizes were observed. |
| Kaca et al. [30] | LAL test | Hemoglobin | Aggregation size decreased | Enhancement of activity was observed. |
| Certain metal ions decrease LPS activity. (LPS aggregate sizes are increased) | ||||
| Tsuchiya [23] | LAL test | Metal ions (Fe, Al, Ga) | Not mentioned | Activity was decreased. |
| Duner [24] | LAL test | Metal ions (Zn, Cu, Fe, Al) | Not mentioned | Activity was decreased. |
| Roth et al. [25] | LAL test, endothelial cell procoagulant activity | Metal ion (Fe) | Not mentioned | Activity was decreased. |
| Fujita et al. [26] | LAL test | Metal ion (Cu, Fe, Al, Ga), antibiotics | Larger aggregates were observed. | Activity was decreased. |
| Fujita et al. [27] | LAL test | Metal ion (Fe) | Cleavage at ester bonds of Lipid A was observed. | Activity was decreased. |
| LER mechanism is explained. | ||||
| Tsuchiya [11] | Review previous literature | Change in LPS aggregation to smaller sizes. | ||
| Reich et al. [7] | LAL test | LER | Mixed micelles containing monomeric LPS | Activity was decreased. |
| Tsuchiya [9] | LAL test | LER | Not mentioned | Effects of temperature, pH salts, and detergent are kinetically analyzed. |
| Reich et al. [31] | LAL test | LER | Aggregates structures are complexed with surfactants. | Simulated LER kinetics is proposed. |
| Tsuchiya [15] | LAL test | LER | LPS aggregates are maintained at low temperature. | Dilution of samples with magnesium solution reflects true activity. |
| *BSA: Bovine Serum Albumin. | ||||
Table 5: List of studies on LPS activity change related to LPS states.
There is a discussion on the difference in LER effects between purified LPS and naturally occurring endotoxin (NOE). NOE is relatively resistant to LER [5,6,9,10]. Structures of NOE are affected by the environment of the growth of the bacteria. The reason of the resistance of NOE to LER is modification of the phosphor groups of LPS [32]. This is reasonable because modification of phosphor groups of LPS with phosphoethanolamine and aminoarabinose can mitigate the effect of chelating agents in LER solutions. However, NOE is not composed of uniform modified LPS structure, and the activity can be decreased under LER conditions at higher temperature [9]. The findings in this study can be partially applied to NOE because NOE includes LPS without modification.
The proposed mechanism of LER is different from previously reported mechanisms of decrement of LPS activity, such as dispersion of LPS molecules and decomposition of LPS. Many of recent biopharmaceutical products contain detergents and chelating agents, and these products may show LER. These products can be required the hold-time study for the Bacterial Endotoxins Test [4,33]. The findings of this study are useful for the investigation of failures of the hold-time studies and considering the strategies to mitigate the LER effect of products.
A new mechanism of LER caused by a chelating agent and a detergent is proposed. Purified LPS forms the non-lamellar cubic structure in water, and the LPS aggregates are reinforced by divalent cations. When purified LPS is added to LER solutions, divalent cations are removed from outer layer of the LPS by the chelating agent, and the loss of divalent cations weakens the outside layer of the LPS aggregates. The LPS aggregation structure is maintained at low temperature, but LPS molecules on the surface of the LPS aggregates are gradually replaced with detergent molecules at higher temperature. This replacement reduces the surface area of the LPS on the LPS aggregates, and causes reduction of activity of the LPS aggregates. The apparent sizes of the aggregates are not changed after the replacement. This mechanism satisfied the findings in this study.
The author is grateful to Dr. Jack Levin and Dr. James F. Cooper for their helpful suggestions and for editing the manuscript.
Citation: Tsuchiya M (2019) Mechanism of Low Endotoxin Recovery Caused by a Solution Containing a Chelating Agent and a Detergent. Immunome Res 15:166. doi: DOI: 10.35248/1745-7580.19.15.166
Received: 03-Apr-2019 Accepted: 25-Apr-2019 Published: 03-May-2019 , DOI: 10.35248/1745-7580.19.15.166
Copyright: © 2019 Tsuchiya M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.