Journal of Cancer Science and Research
Open Access
ISSN: 2576-1447
ISSN: 2576-1447
Case Report - (2023)Volume 8, Issue 2
Medullary carcinoma of the colon is a rare tumor that has a unique and sometimes varied histologic profile, including a solid architecture with poor glandular differentiation, undifferentiated cellular morphology, an aberrant immunophenotype, and DNA microsatellite instability. Patients with medullary carcinoma have a better prognosis than those with other colon malignancies, but this rare tumor is often misdiagnosed. Here we report the case of a 77- year-old woman who presented with gastrointestinal bleeding and a 1-year history of anemia. A right-sided cecal mass was shown to harbor a tumor that had not spread to the lymph nodes. Interestingly, her tumor was positive for expression of Caudal-type homeobox transcription factor 2 (CDX2) and negative for the Keratin 20, which is unusual for this entity. However, overall cellular morphology and DNA microsatellite instability indicated by loss of expression of the mismatch repair proteins MLH1 and PMS2 supported a diagnosis of medullary carcinoma rather than a conventional adenocarcinoma of the colon. A refined understanding of the histologic and genetic as well as clinical phenotypes of medullary carcinoma of the colon is needed to prevent its misdiagnosis as other more aggressive colonic adenocarcinomas.
Medullary carcinoma; Colon cancer; Poorly differentiated adenocarcinoma; microsatellite instability; Mismatch repair
According to the latest Surveillance, Epidemiology and End Results (SEER) Program database, the rate of new cases of colorectal cancer are 37.7 per 100,000 men and women per year. The death rate is 13.4 per 100,000 men and women per year [1]. Data from World Health Organization shows that by the year 2035, the total number of deaths from rectal and colon cancer will increase by 60% and 71.5%, respectively [2]. The most common histologic phenotype of colorectal cancer is adenocarcinoma characterized by glandular architecture [3]. Medullary Carcinoma (MC) of the colon is a relatively rare and new histological variant characterized by a solid architecture, poorly differentiated morphology, an aberrant immunophenotype, and microsatellite instability [4]. It is predominantly a solid tumor with little-to-no glandular differentiation. Overall, this tumor entity is believed to carry a significantly favorable prognosis compared with microsatellite stable poorly differentiated or undifferentiated adenocarcinoma of the colon [5]. Herein, we present a rare case of medullary carcinoma of colon encountered in our daily practice at our institution to shed light on the nuances of this frequently misdiagnosed entity.
77-year old female presented to the emergency department with lower gastrointestinal bleeding along with anemia of 1 year duration. She complained of fatigue but denied any weight loss. Her complete blood count at admission showed hemoglobin to be 8.6 g/dL. The patient was admitted to the hospital and required subsequent transfusions. Peripheral blood smear was positive for microcytic hypochromic anemia. She later underwent a colonoscopy which showed an ulcerative exophytic cecal mass. Right hemicolectomy was performed, and histology showed a high-grade undifferentiated carcinoma consistent with features of medullary carcinoma of the colon (Figure 1A-1E). Surgical resection margins were negative for tumor. Twenty-two lymph nodes also were negative for tumor. The tumor was staged as pT3N0M0. Immunohistochemical stains were positive for caudal type homeobox 2 (CDX2) (Figure 1F) and negative for CK7, CK20 (Figure 1G), synaptophysin and chromogranin, GATA 3, and PAX8. MLH1 and PMS2 showed loss of nuclear expression indicating DNA microsatellite instability (Figure 1H-1K). The patient developed postoperative erythematous, pruritic maculopapular rash on her left hand, and coccyx which may have been an allergic drug rash for which she was treated with hydrocortisone lotion. There were no other surgical complications. She received intravenous famotidine, diphenhydramine, and 125 mg of methylprednisolone. She was also given a prescription for prednisone tapering from 60 mg down to 0 over 10 days. The patient had normal bowel movements during her clinical course (Figure 1).
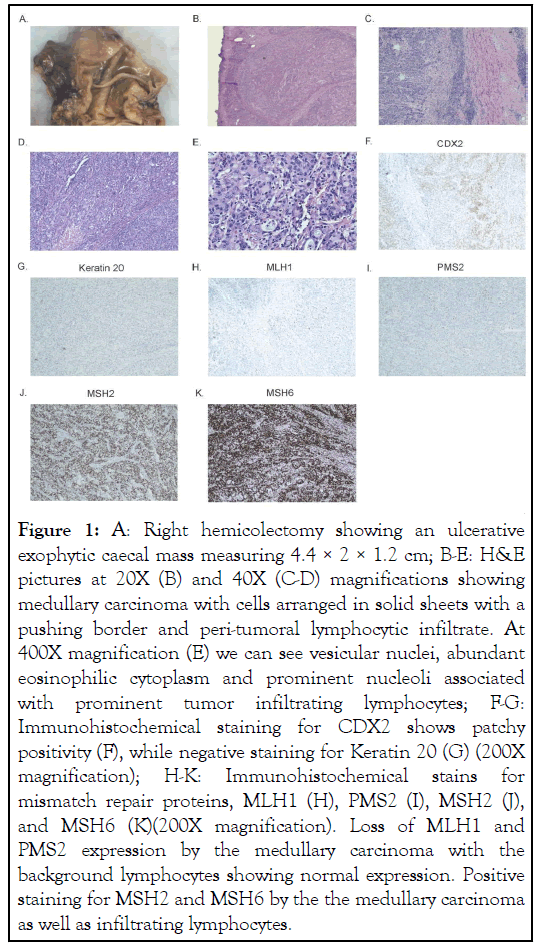
Figure 1: A: Right hemicolectomy showing an ulcerative exophytic caecal mass measuring 4.4 × 2 × 1.2 cm; B-E: H&E pictures at 20X (B) and 40X (C-D) magnifications showing medullary carcinoma with cells arranged in solid sheets with a pushing border and peri-tumoral lymphocytic infiltrate. At 400X magnification (E) we can see vesicular nuclei, abundant eosinophilic cytoplasm and prominent nucleoli associated with prominent tumor infiltrating lymphocytes; F-G: Immunohistochemical staining for CDX2 shows patchy positivity (F), while negative staining for Keratin 20 (G) (200X magnification); H-K: Immunohistochemical stains for mismatch repair proteins, MLH1 (H), PMS2 (I), MSH2 (J), and MSH6 (K)(200X magnification). Loss of MLH1 and PMS2 expression by the medullary carcinoma with the background lymphocytes showing normal expression. Positive staining for MSH2 and MSH6 by the the medullary carcinoma as well as infiltrating lymphocytes.
MC is a rare histologic variant of colon carcinoma, with an estimated incidence of 0.1%-3% of all Colorectal Cancers (CRC) [5]. It was first described by Jessurun J, et al. [6], in 1999 in a study of 11 cases of colon carcinoma with minimal or no glandular differentiation. Only 5-8/10,000 colon cancers have the characteristic histologic feature of medullary carcinoma [7]. Mean age at diagnosis was 69.3 (± 12.5) years, with incidence increasing with age. The mean age at the time of surgery of the MC group was 75yrs. MCs are twice as common in females. They are extremely rare among African Americans. They are most common in the proximal colon (74%), where they present at a later age than the sigmoid colon. Our patient is also a white woman in her 70’s who presented with a right sided cecal mass. MCs were misdiagnosed as poorly differentiated adenocarcinoma NOS in two-third of cases and occasionally for neuroendocrine or metastatic carcinoma in a retrospective review of 302 cases between 1997 and 2018 in a large national health service teaching hospital in United Kingdom (UK). As most poorly differentiated adenocarcinomas behave much more aggressively than medullary carcinoma, neglecting the medullary phenotype could potentially lead to overtreatment, particularly if MMR or MSI testing is not done [8].
Microscopically, MCs are characterized by an undifferentiated morphology with cells arranged in solid sheets with a pushing boarder and having vesicular nuclei, abundant eosinophilic cytoplasm and prominent nucleoli associated with prominent tumor infiltrating lymphocytes and granulocytes. Necrosis is usually present and mitotic activity is rare. It can be either pure or associated with a typical glandular component as described in a case series [4]. Intriguingly, in MCs, CK20 is positive in <50% cases and CDX2 is positive in <30% cases. While both these markers are positive in >70% cases in poorly differentiated adenocarcinomas [8]. According to WHO and also shown in a case report [9], MCs demonstrate an aberrant immunophenotype with loss of CDX2 and CK20. Our case was however focally positive for CDX2 and negative for Keratin 20. Another previous case report had similar findings which was negative for CK20 and positive for CDX2 giving rise to a diagnostic challenge [7]. Other markers of intestinal differentiation like MUC1, MUC2 and TTF can be used to confirm the diagnosis of MC, as they are usually positive for these markers in 67, 60 and 53% of cases respectively, in accordance with a study done by Winn B, et al. [10], using 16 MC cases. However, they cannot be used to differentiate MC from Poorly Differentiated Carcinoma (PDC) of colon as they can be positive in both the entities, with no statistically significant differences. However, three immunohistochemical markers, MLH-1, CDX2 and calretinin demonstrated statistically significant differences in staining (p<0.05) between the MC and PDC groups. MLH1 and CDX2 were positive in 21% and 19% of the MC group as opposed to 60% and 55% of the PDC group (p=0.02 and p=0.03 respectively). The differential staining pattern for calretinin was the most remarkable, with 73% of the MCs as opposed to 12%of the PDCs staining positive [10].
MCs also demonstrate peculiar molecular and genetic profiles in contrast to the regular adenocarcinomas. In a study done by Lanza G, et al. [11], 90% (18 of 20) of the MCs demonstrated microsatellite instability, while 87.8% (36 of 41) were p53 negative by immunohistochemistry and 82 were DNA diploid by flow-cytometric analysis. Interestingly, in a consecutive series of 81 cases, medullary morphology was found in 15% of all MMR deficient tumors and 17% of those showed loss of hMLH1 [8]. In a study by Knox RD, et al. [12], 91 of 3,295 CRCs were medullary type and all of them revealed MMR deficiency and 86% were BRAFV600E-mutated (p<0.0001).
One study showed that several immunoregulatory genes are overexpressed in microsatellite unstable colorectal carcinomas, especially the MC subtype, which may have potential diagnostic and therapeutic implications [13]. MCs can be confused with Lymphoepithelioma-Like Carcinoma (LEL) of the colon which too can be MSI high and associated with prominent lymphoid stroma. However, LEL carcinoma of colon is usually composed of tumor cells arranged in cords, trabeculae, chains, single cells within a dense lymphocytic background while MCs are arranged in solid sheets and characterized by peritumoral and intratumoral lymphocytes. The individual tumor cells are pleomorphic in LEL carcinomas as opposed to the uniform cells noticed in MCs. The border is infiltrative instead of the pushing border seen in MCs [14].
MC points to a better survival as compared to poorly differentiated adenocarcinomas and well to moderately differentiated adenocarcinomas, as has been demonstrated in many clinical studies [11]. According to a study done by Knox et al, from 1998 to 2012, a medullary phenotype was protective (hazard ratio of death 0.54, 95% CI=0.30-0.96; p=0.037) [12].
In conclusion, pathologists require a greater degree of vigilance regarding the possibility of Medullary Carcinoma (MC) when encountering colon malignancies with an undifferentiated phenotype. Awareness of the morphological features of Medullary Carcinoma (MC) and an understanding of mismatch repair and diagnostic immunohistochemical protein staining patterns characteristic of this disease may improve our ability to diagnose this underreported entity with its myriad expressions, which is critical for timely and appropriate treatment.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Saikia K, Bava EP, Chang Q (2023) Medullary Carcinoma of Colon: Pathologists' Dilemma. J Can Sci Res. 8:527.
Received: 06-Jan-2023, Manuscript No. JCSR-23-21277; Editor assigned: 09-Jan-2023, Pre QC No. JCSR-23-21277 (PQ); Reviewed: 23-Jan-2023, QC No. JCSR-23-21277; Revised: 30-Jan-2023, Manuscript No. JCSR-23-21277 (R); Published: 06-Feb-2023 , DOI: 10.35248/2576-1447.23.8.532
Copyright: © 2023 Saikia K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.