Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Review Article - (2024)Volume 12, Issue 3
Abiotic environmental stresses and global climate change have a harsh impact on morphological, physiological, and biochemical characteristics of plants. Further, reduces the growth and development of plants. An indoleamine molecule called Melatonin (MLT) is important for plants' having ability to respond to a variety of abiotic stimuli and lessen their negative consequences. During harsh environmental conditions (such as draught, high temperature, high salinity, and heavy metal pollution), MLT significantly improves plant growth, photosynthetic yield, chlorophyll content, oxidative damage, and enhances the activity of anti-oxidant enzymes such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GOP). In this Review, we discuss that even though plants can biosynthesize MLT and exogenous application of MLT to a variety of crops can be enhance plant development and growth in response to drought stress by controlling the activity of antioxidant enzymes in the plants.
Melatonin; Draught stress; Photosynthesis; Antioxidative stress
From few decades global agricultural productivity is harmed due to climate change. Heat waves, cold waves, floods, and drought situations are caused by abrupt and/or frequent variations in temperature and rainfall patterns [1]. According to 2007 Food and Agriculture Organization (FAO) assessment, 96.5% of the world's cultivated areas experience one or more stress conditions [2]. Furthermore, there will likely be a significant rise in agricultural yield loss caused by abiotic stresses in the near future due to projections that global climate change would accelerate dramatically over the next ten years [3].
It is critical to develop methods to lessen crop damage from abiotic stress brought on by climate change [2]. Some methods being investigated to increase crop plants resistance to abiotic stress include chemical pre-treatments of seeds, the use of microbes, and the development of genetically modified stress- tolerant plants [4].
Oxidative stress is induced in response to abiotic stress. Abiotic stress circumstances that are severe and/or frequent disrupt the cellular redox balance, leading to elevated levels of oxidative stress that may result in permanent harm to the plant [5-7]. Reactive Oxygen Species (ROS) are by products of plant cellular metabolism that include Hydrogen Peroxide (H2O2) superoxide anions, hydroxyl radicals, -OH and singlet (O-1) and triplet oxygen (O-3), [8,9]. Plants have built-in enzymatic and non- enzymatic scavenging systems for ROS detoxification because an excessive build-up of ROS is harmful to the cell. Enzymes include SOD, guaiacol peroxidase, Catalase (CAT), Ascorbate Peroxidase (APX), Thioredoxins (TRX), Peroxiredoxins (PRX), Glutathione Peroxidase (GPX), and Glutathione Reductase (GR) are part of the antioxidant enzyme system [1,9,10]. Avonoids, glutathione, vitamin E, and ascorbic acid are used in non- enzymatic defense processes. Oxidative stress results from the build-up of ROS within the cell when the pace of ROS creation exceeds the rate of detoxification [11]. ROS generation is elevated in response to abiotic stress situations. In trace amounts, ROS aid in signal transduction, enable seed germination, photosynthesis, flowering, and the delay of senescence. They also assist the plant in modifying its metabolism in response to appropriate stress.
Abiotic stress circumstances, on the other hand, throw off the equilibrium between ROS generation and detoxification. When cellular ROS build up above a certain threshold, oxidative stress is initiated, which leads to membrane lipid peroxidation, Malondialdehyde (MDA) production, electrolyte leakage, and eventually programmed cell death. DNA oxidative damage is an additional outcome of cellular ROS build-up. Damage is brought about by breakage in DNA strands and base modifications, which alter cellular functions and coding capacity. Plant growth, development, reproduction, and yield loss are all slowed down as a result of damage from oxidative stress, as well as the breakdown of genetic material and cellular components.
A growing body of research indicates that one of the main factors causing abiotic stress-related harm to plant growth, development, and reproduction is draught stress. According to climate models, droughts will happen more frequently as a result of the long-term effects of global warming. This highlights how vital it is to create agricultural practices that are adaptable to a changing climate.
MLT (N-acetyl-5-methoxytryptamine) is an indolamine and act as an antioxidant and play a significant role in biological rhythm in many phototrophic organisms, [1,4,9-13]. It has been proposed that MLT is not found only in animals but also found in green plants and bacteria. The presence of MLT in plant and their function was firstly discovered by Ahmad [1]. Among the role of MLT in animals, it is also play significant role in physiology of plants such as flowering, scavenging free radicles by regulating scavenging enzymes such as SOD, CAT etc., [9]. Hence, these studies suggest that MLT could be a protectant against abiotic stresses (such as oxidative, draught, salinity) [14-17].
MLT has since attracted the interest of plant biologists due to its widespread distribution, [17] and diverse functions, [14] in plants. The circadian rhythm, photo protection [18], blooming time, seed output, leaf senescence, and fruit ripening are just a few of the many aspects of plant development that melatonin plays a significant role in modulating [2,19-21]. Additionally, it plays a role in plant innate immunity, responses to salt stress [12], low and high temperature, oxidative stress [22], and heavy metal stress, as well as drought and osmotic stress [23].
Within this framework, scientists are always working to increase plant resilience to harsh environmental circumstances. Over the past 20 years, a number of research have been carried out and published indicating that melatonin, an indole base structure, is a crucial potential modulator [14], in plants that increase drought tolerance [24], and plays multiple roles including regulation of water balance, root volumes enhancements, stimulates seed germination, maintains chloroplast, carbohydrate, and proline metabolism, and delays leaf senescence [18,20]. When plants are under drought stress, melatonin has been shown to help maintain the equilibrium of various ions and to regulate the activity of antioxidant enzymes in response to high ROS [22,23,25-27], highlighted the role of melatonin as a growth-promoting agent and antioxidant in oxidative stress. Lately, melatonin treatments increased the activities of antioxidant enzymes in cotton, rapeseed, salvia, flax plant, maize, and buckwheat [18,28,29]. When 100 mm melatonin was applied foliarly to buckwheat during a drought, the plant's soluble sugar and protein content increased by 60%. Similarly, when grape cuttings were treated with melatonin, the proline content of their leaves increased. Another study showed that pre-treatments with melatonin increased osmolyte concentrations like soluble sugars, soluble proteins, and proline in drought stressed soybean leaves [30]. It has been stated that after enhancing their capacity for recovery, melatonin-treated plants make it possible for them to withstand abiotic stress. Since various studies have demonstrated the multifaceted roles and functions of melatonin in stressful environments, it is imperative to formulate a comprehensive concept covering all relevant research regarding the application of melatonin in stressful environments, taking into account its unique pathways and mechanisms within the plant body.
According to research, melatonin acts as a first line of defence and an internal sensor of oxidative stress in plants [31]. For instance, this chemical encourages cucumber seed germination, lateral root development, and drought tolerance [32]. It shields plants against harm brought on by environmental stressors like Ultraviolet rays (UV), temperature changes, and heavy metals. In addition, melatonin slows down the ageing of leaves; it also makes plants more resistant to salinity and cold. It increases the tolerance of plants to salinity and cold [2,8,30,31,32]. This review discusses the involvement of MLT in draught stress tolerance and successful approaches in enhancement of plant growth and efficiency of photosynthesis and antioxidant enzyme activity under draught stress using MLT to minimize draught stress-induced damage in crop plants at different stages of plant development. Furthermore, the biosynthesis of MLT under abiotic stress conditions and biotechnological approaches of MEL for generation of abiotic stress resistance crops are also discussed.
Mechanism of biosynthesis of MLT in plants
MLT has been considered as one of the multipurpose metabolite synthesized in part of the plant body. Usually, MLT involved in vegetative growth, fruit maturation, leaf development etc. However, it has been regularly reported that it is also involved in tolerance of biotic as well as abiotic stresses such as heat, salinity, draught. MLT is synthesized in chloroplast and mitochondria of cells of plant the fact that Serotonin N-acetyltransferase (SNAT), one of the rate-limiting enzymes involved in the biosynthesis of MLT, has been shown to be localised in mitochondria [10,12], and chloroplasts [26,33,34]. In plant and animals, MLT synthesis pathway usually similar. The tryptophan is regarded as precursor organic molecule of MLT. The conversion of tryptophan to MLT is a complex enzymatic process [6,35].A number of plant species have been found to carry the genes encoding all the enzymes involved in the whole MLT biosynthetic pathway, with the possible exception of one gene encoding a Tryptophan Hydroxylase (TPH), which catalyses the conversion of tryptophan into 5-hydroxytryptophan (5HTP) [24,36]. Specifically, this enzyme was only recently postulated in plants. There are at least six enzymes are involved in the synthesis of MLT from tryptophan from different pathway of biosynthesis such as L-tryptophan decarboxylase (TDC), Tryptamine 5-hydroxylase (T5H), Serotonin N-acetyltransferase (SNAT), Acetyl serotonin O-methyltransferase (ASMT), Caffeic acid 3-O-methyltransferase (COMT), and a putative Tryptophan Hydroxylase (TPH) that has not yet been identified are the six enzymes that are known to be involved in the synthesis of MLT.
![]() The synthesis of serotonin from tryptophan during MLT biosynthesis is a prerequisite step. There could be two distinct pathways involved in MLT. In the first method, tryptophan is first converted to tryptamine by TDC. Next, tryptamine is hydroxylated to serotonin by T5H. Alternatively in second method, tryptophan might also be hydroxylated into 5- hydroxytryptophan by TPH, and 5-hydrotryptophan could subsequently be decarboxylated into serotonin by TDC. It has been cleared that decarboxylation occurs more frequently in plants than hydroxylation [1,10,35,36,37].
The synthesis of serotonin from tryptophan during MLT biosynthesis is a prerequisite step. There could be two distinct pathways involved in MLT. In the first method, tryptophan is first converted to tryptamine by TDC. Next, tryptamine is hydroxylated to serotonin by T5H. Alternatively in second method, tryptophan might also be hydroxylated into 5- hydroxytryptophan by TPH, and 5-hydrotryptophan could subsequently be decarboxylated into serotonin by TDC. It has been cleared that decarboxylation occurs more frequently in plants than hydroxylation [1,10,35,36,37].
In the next step, there are three types of enzymes (SNATs, ASMTs, and COMT) involved in synthesis of MLT from serotonin. The first enzyme catalyses and acetylation while the other two enzymes act as methyltransferase. Finally, the serotonin converted into N-acetylserotonin (NAS), and 5- methoxytryptamine, respectively [35,38]. In the final step, N- acetylserotonin, and 5-methoxytryptamine converted into MLT.
In plant cells, the location of the enzymes involved in the production of MLT from tryptophan is varied. While ASMT and COMT are found in the cytoplasm [39], TDC is found in the cytoplasm [40], T5H in the Endoplasmic Reticulum (ER) [41], and SNAT is expressed in chloroplasts [41]. The first and second of the four potential MLT biosynthesis pathways shown in Figure 1 lead to serotonin synthesis in the ER, while the third and fourth pathways produce MLT in the cytoplasmic environment [27]. While SNATs are found in the chloroplast and ASMTs/COMT are only found in the cytoplasm, there are differences in the final subcellular locations of MLT synthesis and accumulation. For instance, MLT is quickly transformed into cyclic 3-Hydroxymelatonin (3-OHM) by the MLT 3- Hydroxylase (M3H) while in chloroplasts melatonin can be metabolised into 2-Hydroxymelatonin (2-OHM) by the MLT 2- Hydroxylase (M2H). In the cytoplasm, serotonin is rapidly metabolised into phenylpropanoid amides, such as feruloyl serotonin, by the Serotonin N-Hydroxycinnamoyl Transferase (SHT) [42]. Plants tend to accumulate high levels of MLT intermediates (such as tryptophan, tryptamine, and serotonin) during senescence or in response to abiotic stressors [43]. As a result, the optimal pathway for biosynthesis starts with tryptophan and goes via the intermediates tryptamine/ serotonin/5-MT and ends with MLT [43], wherein serotonin is converted by COMT into 5-MT via O-methylation, and SNAT acylates it to produce MLT (Figures 1 and 2).
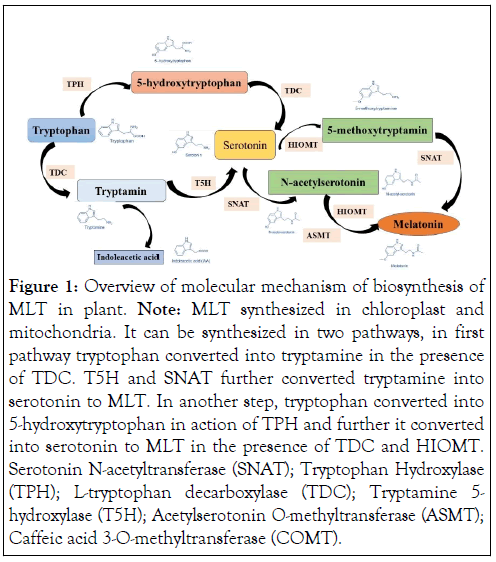
Figure 1: Overview of molecular mechanism of biosynthesis of MLT in plant. Note: MLT synthesized in chloroplast and mitochondria. It can be synthesized in two pathways, in first pathway tryptophan converted into tryptamine in the presence of TDC. T5H and SNAT further converted tryptamine into serotonin to MLT. In another step, tryptophan converted into 5-hydroxytryptophan in action of TPH and further it converted into serotonin to MLT in the presence of TDC and HIOMT. Serotonin N-acetyltransferase (SNAT); Tryptophan Hydroxylase (TPH); L-tryptophan decarboxylase (TDC); Tryptamine 5- hydroxylase (T5H); Acetylserotonin O-methyltransferase (ASMT); Caffeic acid 3-O-methyltransferase (COMT).
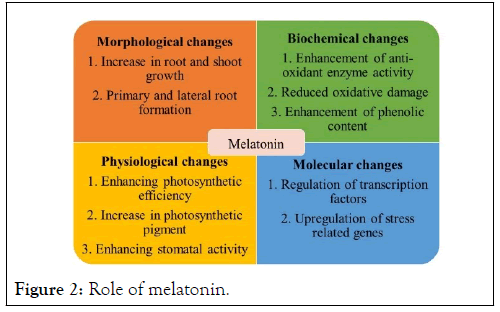
Figure 2: Role of melatonin.
Nevertheless, a large rise in MLT levels was shown to be uncorrelated with a serotonin spike. In fact, in a number of experimental settings, melatonin levels did not rise in proportion to the slight increases in tryptophan and serotonin content that occurred throughout senescence. For instance, research from senescent detached rice leaves have demonstrated that the metabolic capacity of MLT and serotonin synthesis differs by over three times [43]. The very decreased catalytic efficiencies of COMT and SNAT in senescence compared to those under normal growth conditions may account for this significant discrepancy between the two molecules. Nevertheless, compared to N-acetyl serotonin, low quantities of MLT and relatively large levels of 5-MT are achieved. This is despite the fact that serotonin's auto-inhibition of SNATs has not yet been identified, and it is still unclear what additional regulatory roles it plays on the MLT synthesis pathway [44].
MLT levels are unrelated to tryptophan and serotonin levels in cells, even though the MLT biosynthesis pathway produces more MLT under normal conditions than it does during senescence and serotonin boost conditions. Thus, the generation of N-acetyl serotonin by SNAT could be considered the limiting step [45]. In fact, in order for N-acetyl serotonin to be converted to MLT by ASMT or COMT, it must first pass through the chloroplast membrane and enter the cytoplasm [46].
Based on research done on T5H-deficient and T5H-suppressed rice plants, 5-hydroxytryptophan-mediated serotonin production appears to be another possible pathway. MLT levels were higher in the studied plants than in the control plants, whereas serotonin levels were significantly lower. This intriguing finding contradicted the previously established mechanisms for the biosynthesis of MLT [45]. In this instance, it was assumed that a putative TPH oxidises tryptophan to 5-HT, which TDC subsequently transforms into serotonin. The 5-HT pathway is important in increasing MLT levels but does not produce a serotonin boost due to the low levels of serotonin and high amounts of MLT reported in T5H-deficient plants. Lastly, there may be additional pathways for the biosynthesis of MLT [34,47], including ones that do not depend on the production of serotonin. Nevertheless, the enzymes involved have not yet been discovered, and those that are appear not to be engaged in this process.
Efficiency of MLT in plant growth enhancement
The most frequent environmental stress element that lowers plant output is water shortage or drought stress. It causes a morphological, physiological and anatomical disruptions in plants. Furthermore, since a strong root system significantly improves the uptake of immobile nutrients, it is essential for plants to be able to withstand drought stress [48]. A technique for evaluating factors that have an immediate impact on a plant's survival and growth after transplanting is the seedling health index. Crop yield and quality predictions can be made using it [49].
MLT markedly affected the growth parameters (plant height, plant fresh weight) [18], and leaf chlorophyll content of seedlings grown under control. Drought stress also significantly decreased the amount of chlorophyll in leaves and seedling growth (plant height). On the other hand, MLT treatments under drought stress lowered seedling growth (plant height) and leaf chlorophyll content less than untreated plants under drought stress [2].
Liang et al. [2], reported in kiwifruit that draught stress restricted the growth of plant. However, the application of MLT in various concentration increases the plant growth such as in Moringa oleifera, Pisum sativum, Lycopersicum esculantum, Soybean, Dracocephalum moldavica, Cauliflower Plants, Lycopersicum esculantum, Oat, Fagopyrum tataricum Gaertn, Rapeseed, Coffea arabica, Zea mays, Dracocephalum moldavica, Brassica napus, Mung bean and Lycopersicum esculantum [1,14,19,20,22,29,31,50-58]. However, previous studies also demonstrated that there is no significant effect on plant growth parameters under well-watered condition with and without treatment of MLT. Although, under drought stress condition the length of root and shoot, size of leaves also reduced without treatment of melatonin. However, exogenous application of MLT increases root and shoot length and other growth parameters of plants such as Zea mays [32], under draught condition. Recently, it has been reported that application of metal oxide nanoparticles with MLT improves the efficiency of growth parameters in plants. The results proves that application of MLT with TiO2 NPs increase shoot and root growth in Stevia rebaudiana Bertoni [59].Efficiency of MLT in enhancement of photosynthesis
It also effects chlorophyll degradation, photosynthetic yield and rate of leaf senescence. It enhances production of Reactive Oxygen Species (ROS) and if these ROS are not eliminated, resulted in oxidative damage of cell membrane, proteins, and enzymatic activity [60]. Numerous metabolic mechanisms that support water acquisition and/or retention, safeguard chloroplast functions, and keep ions in a balanced state affect how well plants can withstand water shortages [23]. Net photosynthesis decreases under drought stress as a result of regulatory systems that include reducing chlorophyll concentrations, stomatal conductance, and transpiration rates while rising intercellular CO2 levels. Plants respond in a variety of physiological and biochemical ways to drought stress [28]. Dysplasia develops in these circumstances as a result of restrictions on cell division and growth, as well as slower rates of photosynthesis, protein synthesis, and nucleic acid metabolism [61].
Exogenous application of melatonin induces chlorophyll level [62], and increase photosynthetic efficiency [2,14,62], increases in photosynthetic pigment constituent Moringa oleifera [29], Zea mays [8], increases chlorophyll content and stomatal conductance in Cucumis sativus, Moringa oleifera, cucumber and Malus domestica.
Efficiency of MLT in enhancement of antioxidant enzyme activity
Draught stress may lead to harsh impact on crop plants by changing their physiological parameters of plants. It triggers overproduction of atmospheric O2 or causes oxidative stress which is termed as ROS. This ROS may cause negative impact on functions of cell. It also causes lipid peroxidation and cellular damage [63-66]. However, this impact controlled by the production of antioxidant enzymes such as Glutathione (GSH), SOD, Peroxidase (POD), and CAT. The antioxidant system of plant plays a significant role in tolerance of draught stress (Table 1).
| Crop | Response of crops treated with melatonin | References |
|---|---|---|
| Moringa oleifera. | Increases plant growth and photosynthetic pigment constituents | [30] |
| Carthamus tinctorius | Activation of antioxidative enzymes | [63] |
| Oryza sativa | Modulation of anti-oxidative system, osmoregulation | [23] |
| Cucumis sativus | Reduction of MDA and H2O2 reduced electrolyte leakage, high photosynthetic rate enhanced chlorophyll content and stomatal conductance | [9] |
| Zea mays | Enhanced the photosynthetic efficiency by protecting the pigments by degrading, increases the concentration of soluble proteins | [9] |
| Soybean | Enhance functioning of antioxidative system, improved growth and water status | [54] |
| A. chinensis var. deliciosa cv. Qinmei | Increase photosynthetic rate, enhancing chlorophyll synthesis and proline content | [2] |
| Solanum lycopersicum | Enhanced GR enzyme activity declined lipid peroxidation | [23] |
| Chrysanthemum | Improvement in plant water content, improves antioxidant enzyme activity and increases antioxidant substances in leaves | [20] |
| Stevia rebaudiana Bertoni | Increase in shoot and root growth | [63] |
| Moringa oleifera | High photosynthetic pigments phenolic compounds, enhanced IAA | [30] |
| Kiwifruit | Modify root architecture, enhancement of osmoregulation content, increased photosynthetic performance | [30] |
| Fenugreek | Improve growth parameters, enhanced antioxidative enzyme activity and osmo-protectants (proline) and reduced H2O2 and MDA level | [30] |
| Cucumber | Reduced chlorophyll degradation and increase photosynthetic rate and decreased ROS production | [30] |
| Malus domestica | Reduced electrolyte leakage, increased stomatal aperture, enhanced photosynthetic efficiency | [1] |
| Vitis vinifera | Healing of damaged chloroplast ultrastructure, proline content enhanced | [54] |
| Dracocephalum moldavica | Activated superoxide dismutase, ascorbate peroxidase, guaiacol peroxidase | [23] |
| Vaccinium corymbosum | Improvement of water content status, photosynthetic performance and incresase antioxidative enzyme activity | [66] |
| Pisum sativum | Increase in plant growth parameters, photosynthetic efficiency and antioxidative enzymes activity | [1] |
Table 1: Effect of MLT on crops under draught stress.
Recently, MLT has been emerge as a subject of research due to its potent role as anti-abiotic stressor or natural antioxidant. There are several studies on positive effect of MLT on maintaining antioxidative enzyme activity. For example, drought stress significantly raised the contents of GSH and the activity of SOD, POD, and CAT in Dracocephalum moldavica. On the other hand, maize seedlings treated with MLT had higher GSH content and higher activity of SOD, POD, and CAT than untreated seedlings under drought stress.
It has been also reported that MLT-treated plants enhance functioning of antioxidative system such as in cucumber [50], increases antioxidative enzyme activity in Vaccinium corymbosum [62], Zea mays, Chrysanthemum [18] and Carthamus tinctorius [59], reduction in MDA and H2O2 in fenugreek and Cucumis sativus. Although, high concentration of MLT also causes negative impact such as in Zea mays. Additionally, Sarropoulou reported that high doses of MLT may be applied to induce a drop-in chlorophyll content; therefore, an excessive dose of MLT may result in significant harm to structure and function. The dose-dependent method of MLT activity may be the cause of these varying reactions in antioxidant enzymes, and it appears that varying MLT concentrations had significant effects on plant responses. Since antioxidant enzymes are highly susceptible to environmental variations, temperature and precipitation fluctuations, among other climatic factors, have a significant impact on the regulation of enzyme activity. MLT-induced reactions have been shown to be dose-dependent [55].
It has been demonstrated that during drought stress, the application of MLT, enhance the activity of antioxidant enzymes. It is thought that increased plant antioxidant capacity may improve the primary role of MLT in stress tolerance. Applying MLT increased the activity of antioxidant enzymes in tomato under drought stress [14].
Plants under stress exhibit alterations in antioxidant enzyme activity when ROS is eliminated. Therefore, smaller dosages of MLT may enhance the antioxidant system's function and avoid potential oxidative damage [55]. Furthermore, it has been shown that the transcripts of antioxidant enzymes are encoded by these genes. Several studies reveal that MLT might improve draught tolerance by reducing oxidative damage.
Recent published data suggests that exogenous application of MLT significantly increase the antioxidant system activity through the reduction in concentration of H2O2 and MDA under moderate and severe drought stress. It is likely that these effects are achieved via controlling the activity of antioxidant enzymes such CAT, SOD, GPX, and APX.
Plants are able to sustain a higher total chlorophyll content due to reduced oxidative damage and increased hydration status, which leads to an increase in leaf expansion, lateral branch number, and blossom length. Under both mild and severe drought stress, the concentration of MLT had no discernible influence on proline content (an osmo-regulator). Previous studies suggest that MLT primarily mitigates the effects of drought stress in Moldavian balm plants by triggering their antioxidant defence mechanism, as opposed to other regulatory channels like osmo-protection and proline content.
New insights into the direct and indirect mechanisms of MLT activity in plants will come from more research focusing on the specifics of the methodology and clarifying the impact of exogenous MLT on modifications in the particular molecular and biochemical pathways.
Thus, it is clear from the above information that MLT alleviates draught stress in plants by activation of anti-oxidative enzymes. It has been also clear that MLT enhancing modulation of anti- oxidant enzymes under draught stress condition to reduce cell damage. Although, the high concentration of MLT can negatively impact the enzymatic activity of anti-oxidant enzymes.
Effect of MLT on SOD activity: Abiotic stresses, including drought, salinity, heat, heavy metals, and oxidative stress, induce the activation of Superoxide Dismutase (SOD). Existing literature demonstrates that the external application of Melatonin (MLT) significantly enhances SOD activity in response to various stressors such as heavy metal exposure, salinity, heat, cold, and drought. Consequently, it is evident from these studies that the upregulation of SOD expression is directly associated with MLT, exerting a significant impact on SOD activity. In a recent investigation, pre-treating tomato plants with 50 or 100 µm of melatonin notably increased SOD activity under salinity stress [54]. However, SOD activity was comparatively lower in naked oat seedlings treated with melatonin than in untreated seedlings. Other researchers, have also observed elevated SOD activity during salinity stress, positively influencing plant development. Similar reports of increased SOD activity have been documented under conditions of waterlogging, alkaline stress, heavy metal exposure, drought, and chilling stress. After subjecting Coffea arabica plants to drought followed by rehydration [55], found a decrease in SOD activity. Conversely, in Melissa officianalis, the addition of melatonin with Cd and Zn resulted in increased SOD activity. This suggests that pre-treating crop plants with melatonin enhances SOD activity, thereby improving their tolerance to various abiotic stresses. The specific mechanisms by which melatonin regulates antioxidant enzymes remain unknown. Nonetheless, studies have consistently shown nano molar gene expression in melatonin-treated cells. Numerous plant studies have reported the overexpression of distinct SOD isoforms, such as ZnSOD and FeSOD, following exogenous melatonin administration. According to previous studies, melatonin treatment during combined stress (high salinity and heat) led to increased expression of SOD (Cu/ZnSOD, FeSOD) compared to control plants [24]. The available research suggests that applying exogenous melatonin may enhance SOD activity in challenging environments. However, the variability of SOD activity under abiotic stress depends on factors such as plant species, plant sections, applied concentration, and duration of exposure. Thus, ongoing fundamental research is essential to better understand this complex phenomenon.
Effect of MLT on CAT activity: Catalase (CAT) serves as a primary defense mechanism for plants in coping with diverse abiotic challenges, aiding in the regulation of healthy Reactive Oxygen Species (ROS) levels. However, the response of CAT to various abiotic stresses appears to be contingent on factors such as the duration, severity, and nature of the stress, leading to conflicting findings in several investigations [56,63,64].
Research has delved into the impact of exogenous melatonin on CAT activity in the context of abiotic stress, revealing varied effects ranging from increased, decreased, to no discernible impact on CAT activity. For example, observed a substantial increase in CAT activity in Zea mays under salt stress with the application of exogenous melatonin (20 and 100 µm) [54]. Similarly, Das and Roychoudhury found elevated CAT levels in Cynodon dactylon under salt stress with melatonin administration (300 mm), proposing that melatonin mitigates cell damage induced by heightened ROS during salt stress by enhancing CAT activity.
In response to waterlogging stress, it has been noted a significant increase in CAT production under melatonin treatment, restoring CAT activity to control levels with a 200 µm melatonin spray. It has been reported higher CAT levels under drought stress, with similar effects observed under acid rain, cold, and vanadium stress [24]. Melatonin therapy also demonstrated the ability to boost CAT activity in combined stress situations, including lead and acid rain stress, salinity and heat stress, and salinity, drought, and cold stress. However, a decrease in CAT activity when melatonin (100 µm) was applied to wheat seedlings under cadmium stress.
Contrasting results were found by Turk, where CAT activity remained constant, and wheat seedlings subjected to cold stress exhibited increased levels of SOD, GPX, APX, and GR. Similarly, it has been reported a similar outcome in Cucumis sativus. under chilling stress. Various studies explored CAT gene expression to corroborate the impact of melatonin on CAT activity in severe settings, revealing consistent outcomes with the levels of functional protein within the cell. The study demonstrated a significant upregulation of the CAT gene in Cucumis sativus. under extreme salt stress (150 mm) with exogenous melatonin treatment (1 µm). Additionally, it has been observed that increased CAT gene expression under oxidative stress and vanadium exposure [50].
However, Martinez, reported a downregulation of CAT gene expression in Solanum lycopersicon. under combined stress conditions, including salt and heat stress, with melatonin treatment (100 µm). These findings suggest that melatonin aids plants in overcoming abiotic stress by modulating CAT gene expression in a stress-specific manner. Overall, applying exogenous melatonin to various crop species under diverse abiotic stressors generally results in increased CAT activity. Nevertheless, contradictory findings from some studies suggest that CAT may not consistently respond to melatonin administration, emphasizing the need for further research to comprehensively understand the interaction between melatonin and CAT activity in the presence of abiotic stressors.
Effect of MLT on GPX activity: Both transcriptional and post- transcriptional levels of the antioxidant system's components are upregulated under hostile environments. GPX and CAT are the main antioxidant enzymes that plants use to scavenge Reactive Oxygen Species (ROS). More and more research has shown that GPX in plants regulates how they react to a variety of abiotic stimuli, including heat, cold, salt, drought, and oxidative stress. Similar to other antioxidant enzymes, functional GPX levels in cells typically rise steadily over time. As a result, numerous studies have shown how exogenous melatonin affects GPX activity under different abiotic stress scenarios. Melatonin usually increases GPX levels in plants by controlling the expression of genes linked to antioxidants. It has been recently showed that exogenous melatonin (20 and 100 µm) application under salinity stress significantly increased the activity of GPX in Zea mays [42]; however, this study also suggested that melatonin (20 and 100 µm) application did not increase the levels of functional GPX at the cellular level under normal conditions. Recent studies, detailed how exogenous melatonin affects cellular GPX function [56].
Moreover, Martinez observed a heightened activity of Glutathione Peroxidase (GPX) in Solanum lycopersicon plants subjected to a combined stress of salinity and heat (35℃+75 mm NaCl) after pre-treatment with melatonin (100 µm). Numerous investigations have delved into GPX transcript levels in an attempt to bridge knowledge gaps regarding the specific influence of melatonin on GPX regulation at the RNA level in response to diverse stressors. They noted the upregulation of the GPX gene responsible for maintaining low peroxidase levels in Solanum lycopersicon. following exposure to elevated salt levels and heat, with exogenous melatonin treatment (100 µm). This finding suggests a significant regulatory role of melatonin in modulating GPX gene expression.
Additionally, it has been reported that an augmentation in the relative expression of GPX in plants exposed to vanadium stress. These outcomes tentatively indicate that melatonin treatment may enhance GPX gene expression in plants. However, this aspect remains underexplored, with limited studies investigating it [56]. These findings suggest that pre-treatment with melatonin has the potential to elevate cellular levels of functional GPX activity across various abiotic stressors, as documented in roots in a single study thus far.
Furthermore, given the presence of multiple orthologous genes for this enzyme in most plant species, a more comprehensive investigation is imperative to understand the genome-wide expression of GPX genes.
Mechanism of MLT mediated draught stress alleviation
MLT can be used to reduce draught stress in various crop plants such as vegetables, cereals etc., it has been reported that MLT act as an efficient molecule against the detrimental effect of draught stress on plant growth and development [19,65]. It can be simply applied and efficiently done by various type of practices including, foliar application, soil treatment, seed treatment etc., to tolerate draught stress. Previous studies illustrated that MLT functionally act in draught stress tolerant in various plants rice, barley [11], bermudagrass [17], and Arabidopsis [66].
Research has demonstrated that melatonin under stress can facilitate the multi-fold biosynthesis of enzyme activities. This has been demonstrated by studies on anatomical changes, such as reduced membrane damage and more intact chloroplast grana lamella; mitigation of leaf active structural damage and preservation of its assembly, protection of mitochondrial assembly [17], maintenance of cell expansion, leaf thickness, and stomata size [28], cuticle cell formation and wax accumulation. Melatonin, which is produced by cell organelles, will persist as long as the severity of the drought grows.
In order to prevent oxidative damage, it activates the physiological antioxidant system. This results in reduced reactive nitrogen and oxygen species accumulation, enhanced nitro- oxidative homeostasis, decreased lipid peroxidation [57], decreased electrolyte leakage and relative water conductivity [39], lowering the toxicant content, limiting cellular redox disruption [14] and increased Ascorbate-Glutathione (AsA-GSH) cycling capacity [54]. These positive outcomes are attained by managing osmoprotectants, enzymatic and non-enzymatic antioxidant activity, and certain secondary metabolites, such as flavonoids, phenols, and phenylalanine ammonia-lyase [8,19,51].
Meanwhile, greater levels of chlorophyll [2], photosynthesis [32], and transpiration rates [55], indicate that melatonin enhances the photosystem of plants. Furthermore, by increasing photosystem II efficacy, melatonin effectively improves photoprotection. Melatonin is a multifunctional molecule that affects cellular osmotic potential through the accumulation of soluble carbohydrates and proline [57].
Furthermore, melatonin's first goal for reducing drought is water modification. In this sense, melatonin helps plants because it is assumed that when they experience drought or water scarcity, melatonin's regulatory and protective functions will kick in at the same time as other likely anti-stress mechanisms to offset the negative effects of stress [3], research on melatonin applications in field crops with respect to drought is incredibly rare.
Studies on the fertility of anthers during drought and the application of melatonin to cotton seeds for germination [42], have been done, but they have not shown how beneficial melatonin concentration is for the physiological development of the leaf over time. Recent studies, treated drought-stricken cotton plants with melatonin doses 335 Days After Flowering (DAF). Their findings demonstrated the effectiveness of foliar melatonin treatment in suppressing boll shedding and promoting physiological characteristics of cotton leaves linked to antioxidant defence systems and sugar metabolism.
The common mechanism of drought and the melatonin treatment's anti-stress response for leaf physiological features are explained in detail in the schematic picture below. Overall, the research that have been published offers strong support for the hypothesis that melatonin can help crops that are experiencing drought stress. Nevertheless, the precise mechanisms via which melatonin reduces drought stress in various plants are intricate and may entail a number of pathways. By utilising optimal melatonin concentrations in various crops and environmental settings, future study may enable a better understanding of the symmetry of the underlying systems and the creation of ways to improve crop resilience against drought stress.
MLT induced signal transduction pathway and metabolites under draught stress
Global climate change leads to changing in environmental factors, biotic as well as abiotic. Abiotic stress such as salinity, draught, heat and heavy metal have profound impact on morphological, physiological, biochemical and molecular parameters of plants [1,10]. Among these, draught stress causes harsh impact on plants which resulted in increase in oxidative stress [22], with detrimental impact on cellular disturbances, cell permeability, photosynthetic yield [19], and other developmental parameters. In response to tolerance of these stresses such as in draught stress, MLT play significant role [26]. Previous studies on role of MLT signalling in stress responses provide further research on role of MLT in other abiotic factors. During the exposure of stress, MLT function in six steps including; stimulation of activity of antioxidant enzyme, enhancement of stomatal activity, reduction in accumulation of toxic substances, activation of defence genes, act in secondary metabolite accumulation, and modification of cell wall. MLT act as signalling molecule via a MAPK pathway. It has been reported that, Cullin Associated and Neddylation Dissociated 2 (CAND2) is regarded as a MLT receptor found in either plasma membrane or cytoplasm. MLT receptor require activation of MAPK, a Mitogen-Activated Protein Kinases (MAPK) cascade. MLT firstly, activates MAPK cascade [26,67]. MAPK cascade induces translocation of Natriuretic Peptide Receptor 1 (NPR1) a salicylic acid receptor into nucleus. After it interact with pathogen resistance transcriptional factors Presenilin-1 (PS1) and Isochorismate Synthase 1 (ICS1). The exogenous application of MLT interact with MLT receptor and induces MAPK3/6 cascade by activation of MAPK. Further, MAPK3/6 cascade induces the transcription factor, basic Leucine Zipper (bZIP60) which is responsible for expression of Binding Immunoglobulin Protein (BIP2, BIP3) and Calnexin (CNX1) genes. These genes (BIP2, BIP3 and CNX1) are responsible for attenuation of stress damages endoplasmic reticulum. Under the draught stress condition, ROS burst occurs from mitochondria, chloroplast and respiratory burst oxidase homologs. During, draught stress condition also induces synthesis of MLT metabolites. ROS are responsible for induction of MLT metabolites biosynthesis such as N1-Acetyl- N2-formyl-5-methoxykynuramine (AFMK), N-Acetyl-5- methoxykynuramine (AMK), 5-Methoxytryptamine (5-MT), 2- Hydroxymelatonin (2-OHM), cyclic 3-Hydroxymelatonin (3- OHM). These MLT act as significant antioxidant. The induction of MLT metabolites, function scavenging of ROS generating during draught stress. shown that melatonin facilitated SGT1- mediated signals to mitigate drought stress. These signals were likely mediated by ABI5-mediated production of antioxidant genes, such as OsCAT2, which may facilitate the scavenging of Reactive Oxygen Species (ROS) until ROS homeostasis is reached [6]. On the other hand, during MLT signalling in draught stress condition, MLT crosstalk with plant hormones to tolerance of draught stress. The low concentration of MLT can induces biosynthesis of Indole-3-acetic acid (IAA) auxin. It also inhibits expression of IAA related factors and play important role in tolerance of draught stress such as inhibition of leaf senescence [10]. Under water harsh condition or draught stress, the stomatal activity of plants also affected. MLT up-regulates expression of Abscisic Acid (ABA) genes and affects signalling of ABA which further affects stomatal behavior in response to draught stress condition. It has been reported in previous studies that application of MLT induces the accumulation of Jasmonate (JA) and Salicylic Acid (SA) under draught stress condition in plants. Also, under biotic stress or in plants infected by pathogen. However, mutation in SNAT decrease the biosynthesis of MLT [53].
Biotechnological approaches of MLT for generation of abiotic stress resistance crops
It has been confirmed that in agricultural production, the MLT play vital role in biomass quantity, photosynthetic yield, improvement of activity of antioxidant enzymes under draught stress. However, how MLT function and what is the mechanism under stress condition is not known. Recently, biotechnology open a new field to improve stress resistance plants [60]. Previous studies reported that, the biotechnological approaches such as genomics, transcriptomics, metabolomics and proteomics can be used in the physiological and molecular mechanisms of adaptation under various stress condition. Therefore, with the help of contemporary breeding and biotechnological tools, the MLT impact may be thoroughly investigated. The knowledge thus produced can then be applied to enhance plant response to various stress situations [10]. It is also possible to study the signalling of abiotic stress resistance and interaction between MLT and phytohormones of plants such as ABA, Gibberlic Acid (GA), IAA. Although, the interaction between MLT yet not been studied well [12]. It has been clear that the advanced Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology can play a vital role in gene editing and development of stress tolerant plant [11]. However, there are need more research in MLT biosynthesis.
This is clear that remarkable development on MLT research in plant has been made. There are no any type of gland for biosynthesis of MLT thus, the biosynthesis pathway is different from animal in plants. The biosynthesis pathway of Melatonin (MLT) in plants shares similarities with auxin biosynthesis, with only a few differing enzymes. Numerous studies have demonstrated the important role of MLT in improving various plant growth parameters and enhancing photosynthetic efficiency under both biotic and abiotic stress conditions.
Author would like to thanks Plant Bioenergetics and Biotechnology Laboratory, Department of Botany MLS University, Udaipur, Rajasthan (India) for providing essential facilities.
Not applicable.
All authors are agreeing for publication.
All authors are participated in this research work.
VS conceived the idea; YS wrote the whole manuscript. The complete manuscript was revised and edited by GS and UB, supervised by VS.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The author declare that they have no competing financial interest or personal relationship that could have appear to influence the work reported in this paper.
The data and materials that support he findings of the study are available from the corresponding author upon request
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Sompura Y, Shah G, Bhatt U, Soni V (2024) Melatonin Mediated Enhancement of Photosynthetic Efficiency and Antioxidant Activity under Drought Stress. J Plant Biochem Physiol. 12:344.
Received: 15-Mar-2024, Manuscript No. JPBP-24-30165; Editor assigned: 19-Mar-2024, Pre QC No. JPBP-24-30165 (PQ); Reviewed: 02-Apr-2024, QC No. JPBP-24-30165; Revised: 09-Apr-2024, Manuscript No. JPBP-24-30165 (R); Published: 16-Apr-2024 , DOI: 10.35841/2329-9029.24.12.294
Copyright: © 2024 Sompura Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.