Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2019)Volume 9, Issue 6
High-protein diets are popular for weight management because of their ability to enhance satiety but not all protein sources are similarly anorexigenic. We recently reported that egg white protein is more satiating than wheat gluten in Sprague Dawley rats. The goal of this study was to identify physiological correlates of the satiety differences and to test long-term consequences on metabolism, body composition and body weight. At both 20% and 35% levels, egg white increased total plasma amino acids relative to wheat gluten. Insulin levels were unaffected but respiratory exchange ratio was reduced for several hours following 35% egg white relative to the other diets. No chronic effects on body weight, body composition, energy intake, or energy expenditure were observed. Results suggest that increased satiety-signaling amino acids may underlie greater short-term satiety from egg white, but when consumed only once per day, produces no long term consequences for body composition.
Protein; Egg white; Wheat gluten; Satiety; High-protein diet; Amino acids
Emerging evidence suggests that the source of protein can influence the satiety enhancing effects of a high protein diet. For example, whey protein decreases subsequent food intake relative to an isoenergetic diet with casein as the protein source in both humans and rodents [1-10]. Ratings of appetite and subsequent food intake also appear to differ following test meals containing soy, pea, tuna, turkey, and egg protein sources [7,11-13]. Furthermore, we have previously observed that rats given 35% egg white protein as the first meal of the day were more satiated than when provided a 35% wheat gluten protein meal [14]. However, the underlying physiological mechanisms explaining the differential effects of the alternate sources of protein remain unknown.
One mechanism that could potentially explain differences in satiety between egg white and wheat gluten is the amino acid profile in circulation following protein ingestion. In addition to each protein source having its own unique amino acid composition, various additional factors are involved in the digestion, absorption, and utilization of dietary proteins and amino acids, which will determine amino acid availability following a protein meal [15-17]. In particular, wheat gluten protein is composed of higher levels of glutamine, most of which is utilized by first-pass metabolism in intestinal enterocytes and hepatic tissues [16,18,19]. However, increased levels of glutamine in wheat gluten protein comes at the expense of essential amino acids [20] which effectively results in an overall lower level of essential amino acids in wheat gluten in comparison to egg white protein. Therefore, it is reasonable to hypothesize those higher levels of circulating essential amino acids following egg white protein intake may trigger more effectively feedback signalling of energy sufficiency [21,22].
The different amino acid profiles resulting from the different protein sources might also differentially impact the release of satiety-stimulating hormones such as cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), peptide YY (PYY), ghrelin and insulin [23-26]. We focused on insulin in this study because previous studies have found positive correlations between insulin levels and satiety [12,27,28] and other studies found protein sources affect insulin release [29-34]. We hypothesized that insulin levels would be higher in egg white- vs. wheat gluten-fed rats, and that the individual differences in insulin levels would be associated with higher postprandial plasma levels of amino acids, leucine, isoleucine, valine, phenylalanine, and arginine [35,36].
Proteins and amino acids consumed in excess of protein deposition cannot be stored in the body, and have to be metabolized shortly after ingestion [37]. Energy expenditure for ATP synthesis from amino acids is known to vary in energetics, ranging from 99.2 kJ/ ATP (for glutamate) through 153.2 kJ/ATP (for cysteine) [38]. Egg white protein contains higher concentrations of amino acids that require more energy to metabolize as compared to wheat gluten protein. Therefore, higher energetics associated with oxidizing amino acids from egg white protein can drive up diet-induced energy expenditure, which through the combined effect of increased oxygen consumption and body temperature, could contribute to enhanced satiety [39].
The goal of the current study was twofold. The first goal was to determine whether the source of protein, egg white or wheat gluten, fed in a high protein diet (20% and 35%), influences plasma amino acid levels, insulin, and metabolism when fed as the first meal of the day in male and female rats. The second goal was to determine whether protein source differentially affects metabolism, body mass, and body composition when fed at the first meal of the day for 30 consecutive days. We designed the study so that all the treatment diets were isoenergetic, with the difference made up of carbohydrate, keeping fat constant. Each of the diets were formulated with only one source of protein (egg white or wheat gluten) rather than mixed in order to methodically gain insight on the effects of each protein source on its own. While 35% protein is within reason for a high protein meal it is at the high end for human consumption. A protein level of 20% is more typical in individuals trying to maintain a high-protein diet [40].
Animals and general husbandry
Sprague Dawley rats were used. See individual experiments for vender, sex, age, and sample size information. Rats were always kept in a temperature-controlled room (26 ± 2°C) with a 12 h reverse light cycle (light on at 20:00 h), and were always provided ad libitum access to water. When not fed the experimental powdered diets (see below) rats were given ad libitum access to powdered AIN93M (Research Diets, New Brunswick, NJ). All studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois and are in accordance with the Guide for Care and Use of Laboratory Animals (National Research Council).
Experimental diet preparation
Treatment diets were prepared to be comparable to AIN-93G. All diet components were purchased from Dyets, Inc. (Bethlehem, PA), with the exception of egg white powder and maltodextrin which were purchased from Harlan (Indianapolis, IN). Four diets were compared in a 2 × 2 design by manipulating protein level (20% or 35%) and protein source (egg white or wheat gluten) (Table 1). The 35% protein diets were approximately 45:35:20 (carbohydrate:protein:fat, as a % of calories) whereas the 20% protein diets were 60:20:20 (Carbohydrate: Protein: Fat, as a % of calories). These diets will hereafter be referred to as 20EW (20% egg white), 20WG (20% wheat gluten), 35EW (35% egg white), 35WG (35% wheat gluten). Separate mineral mixes were used to account for micronutrient differences in the egg white powder and wheat gluten powder. The criteria for the levels of protein used were the following: i) above the recommended amount of protein intake (12- 18%) in both humans and rodent diets; ii) 20% protein reflecting the 90th percentile of protein intake within the population and the typical recommended level in high-protein diets in humans, whilst iii) the 35% was included to reflect the upper-limit of high-protein diets in humans [40]. In regards to the other macronutrients, in order to keep the diets isoenergetic changes in levels of protein needed to be paralleled by changes in carbohydrates, keeping the fat levels constant, as previously recommended [14].
| Grams | 20EW | 35EW | 20WG | 35WG |
|---|---|---|---|---|
| Egg White Powder | 246 | 430 | -- | -- |
| Wheat Gluten Powder | -- | -- | 262 | 460 |
| Corn Starch | 377 | 261.4 | 367 | 242.6 |
| Malt dextrin | 121.6 | 84.3 | 118.4 | 78.2 |
| Sucrose | 101.4 | 70.3 | 98.6 | 65.2 |
| Cellulose | 53.5 | 53.5 | 53.5 | 53.5 |
| Soybean Oil | 90 | 90 | 90 | 90 |
| Mineral Mix (EW) | 35 | 35 | -- | -- |
| Mineral Mix (WG) | -- | -- | 35 | 35 |
| Vitamin Mix | 10 | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| L-Lysine | -- | -- | 3.9 | 6.8 |
| L-Threonine | -- | -- | 1.0 | 1.8 |
| Kcal% | -- | -- | -- | -- |
| Protein | 20% | 35% | 20% | 35% |
| Carbohydrate | 60% | 45% | 60% | 45% |
| Fat | 20% | 20% | 20% | 20% |
EW: Egg White; WG: Wheat Gluten
Table 1: Diet composition.
Feeding schedule
In all three experiments, rats were entrained to the same feeding schedule. Food was removed one hour prior to the onset of the light cycle for an overnight fast. At one hour into the dark cycle (9:00 h), animals were given 30 min to consume a “test” meal, representing 10% average daily intake by weight (average of food intake on previous 3 days). Food intake during the test meal was closely monitored so that feeders were manually opened at one hour after the start of the dark phase and were closed as soon as the allotted 10% of average daily intake was consumed or the 30 min meal period was over, whichever event came first. At 13:30, rats were given ad libitum access to powdered AIN93M until 19:00 h when the cycle continued.
Experiment 1: Food intake and metabolism following test meal
Male (n=12) and female (n=12) Sprague-Dawley rats (4 months; Envigo, Indianapolis, IN) were individually housed in conventional rat cages (48 × 27 × 21 cm) for 7 days before being transferred to the Comprehensive Laboratory Animal Monitoring Systems (CLAMS) (Oxymax; Columbus Instruments International, Columbus, OH, USA) cages. Rats were allowed to acclimate to being housed in the CLAMS cage for two days before data was collected. A within subjects design was used where all rats were given all the treatments in counterbalanced order. However, in some cases, a test diet was not consumed completely, resulting in the following data points removed: 3 males and 5 females in the 35EW treatment group and 2 females from the 20EW treatment group. The total sample sizes are as follows: 20WG (n=12 for male and females), 20EW (n=12 for males and n=10 for females), 35WG (n=12 for males and females), and 35EW (n=9 for males and n=7 for females). CLAMS was used to continuously measure duration and amount of food intake, as well as collect indirect calorimetry data from measured carbon dioxide production and oxygen consumption at 13 min intervals for 23 h periods. On non-testing days, rats were provided ad libitum powdered AIN93M for the whole day according the feeding schedule. On testing days, rats were fed according to the feeding schedule above except that feeders were reopened for rats to access powdered AIN93M at 10:00 h to measure subsequent food intake. Meals were defined as being the total of all bouts in which consumption was greater than 0.03 g and with the time between each bout being no longer than 10 min. Feeding and metabolic responses were assessed following ingestion of test meal on alternating days until each rats received each of the four test diets. Rats were given a day between testing data collection days in order to avoid learned anticipation of food access following testing meal and to provide a washout period. On these intermediate days, rats were provided powdered AIN93M during the first meal period and ad libitum food intake was restricted to be between 13:30 and 19:00 h.
Respiratory exchange ratio (RER) was calculated as VCO2 production / VO2 consumption. Heat production was calculated using the following equation:
Heat = 3.815 + 1.232 × VCO2
Experiment 2: Postprandial plasma amino acid and insulin
Male Sprague-Dawley rats (~200 g; Charles River Laboratories) were individually housed in plexiglass cages (30 × 30 × 38 cm) in which the floors of the cages that were fitted with steel wired bottoms resting approximately one inch above the cage floor in order to measure diet spillage. Plasma samples were collected as previously described [41] from rats adapted to the feeding schedule and fitted with jugular vein catheters. Rats were retrained to the feeding regimen for at least 4 days following recovery from surgery and before samples were collected on experimental days. The following were final sample sizes for each treatment diet (20EW, n=6; 20WG, n=6; 35EW, n=6; 35WG, n=7). Unequal sample sizes are due to complications from the surgery or maintaining patency of the catheters. A total of six samples were taken every 30 min, with the first sample taken 15 min before the test diet was given and the final sample taken 120 min (2 hours) after the test diet was given. Approximately 250 μL of whole blood were collected at each time point and aliquoted into heparinized microtubes for amino acid analysis and into EDTA coated microtainers for insulin analysis. Red blood cells were re-suspended in an equal volume of 0.9% sterile saline solution and returned to each animal through the jugular vein catheter. Plasma samples were kept on ice until stored at -80°C until amino acid analysis.
Amino acids were analyzed by HPLC with fluorometric detection [42] using an Ultimate 3000 RSC System (Thermo Scientific). Plasma was deproteinated with 3.5% perchloric acid and centrifuged for 10 min at 6000 xg before injection onto a 250 × 4.0 mm C18 (5 μm) column after derivatization with o-phthaldialdehyde. Amino acids were separated using a binary gradient with sodium phosphate and acetonitrile/methanol based buffers. Data were collected and analyzed using Chromeleon Data System software (Thermo Fischer). Plasma insulin levels were determined using a rat insulin ELISA kit (EXRMI-13K, Billerica, MA). Samples were analysed in duplicates. Acceptable coefficient of variance for plasma insulin was less than 15%.
Experiment 3: Chronic diet intervention
Male (n=6/treatment) Sprague-Dawley rats (4 months; Envigo, Indianapolis, IN) were individually housed in conventional rat cages (48 × 27 × 21 cm) for 7 days before being transferred to CLAMS. Rats received the feeding regimen with the same treatment meal at the first meal of each day for 30 days continuously. Food intake and body weight was monitored daily. Indirect calorimetry data were collected for the initial 3 days and final 3 days of the 30-day intervention. Data were averaged for each 3 day period for statistical analysis. Body composition (lean and fat mass) was evaluated by EchoMRI (EchoMRI Medical Systems, Houston, TX) at baseline (Day -2), middle (Day 15), and immediately after the end of the study (Day 31).
Statistical analysis
Data were analyzed using SAS v9.4 (SAS Institute Inc., Cary, NC). Food intake was analyzed by a mixed model three-way analysis of variance (ANOVA) with main effects of sex (between subjects; male or female), protein source (within subjects; wheat gluten or egg white), and protein level (within subjects; 20% or 35%). In these models, individual was entered as a random effect to account for the repeated structure. Changes in body weight and body composition were analyzed by repeated measures ANOVA with date as the within-subject factor and treatment (20EW, 20WG, 35EW, 35WG) as the between-subject factor. Changes in RER and heat were analyzed by repeated measures analysis with time as the within-subject factor and treatment (20EW, 20WG, 35EW, 35WG) as the between-subject factor. Post hoc tests analyzed individual time points using 2-way ANOVA with protein level and protein source as factors. Concentrations of amino acids were analyzed by repeated measures analysis with time point (6 levels) as the within-subject factor and treatment (20EW, 20WG, 35EW, 35WG) as the between-subject factor. When treatment effects were detected without an effect of interaction of treatment and time point, data were collapsed across time-points and these values were analyzed by 2-way ANOVA with concentration (20% or 35% protein), type (egg white or wheat gluten), and their interaction as factors. Tukey’s post hoc analyses were used to evaluate pair-wise differences between means. Values were presented as means ± SEM. An alpha level of P<0.05 was considered statistically significant.
Experiment 1: Food intake and metabolism following test meal
Subsequent meal size differed between treatment groups (F1, 78=9.25, P<0.005) (Figure 1A). Rats provided meals containing egg white protein had decreased food intake at the subsequent meal compared to wheat gluten fed rats (P=0.003). Average subsequent meal size following egg white was 2.81 g± 0.257 (SE), whereas for wheat gluten it was 3.85 ± 0.224 g. No effect of protein level on subsequent meal size was detected, or interaction between level and source. Neither protein level nor source affected the food intake for the remainder of the day (Figure 1B). As expected, males ate more than females during the first meal (F1, 78=43.17, P<0.001) and remainder of the day (F1, 78=43.02, P<0.001). No interactions between sex and level or source of protein were observed.
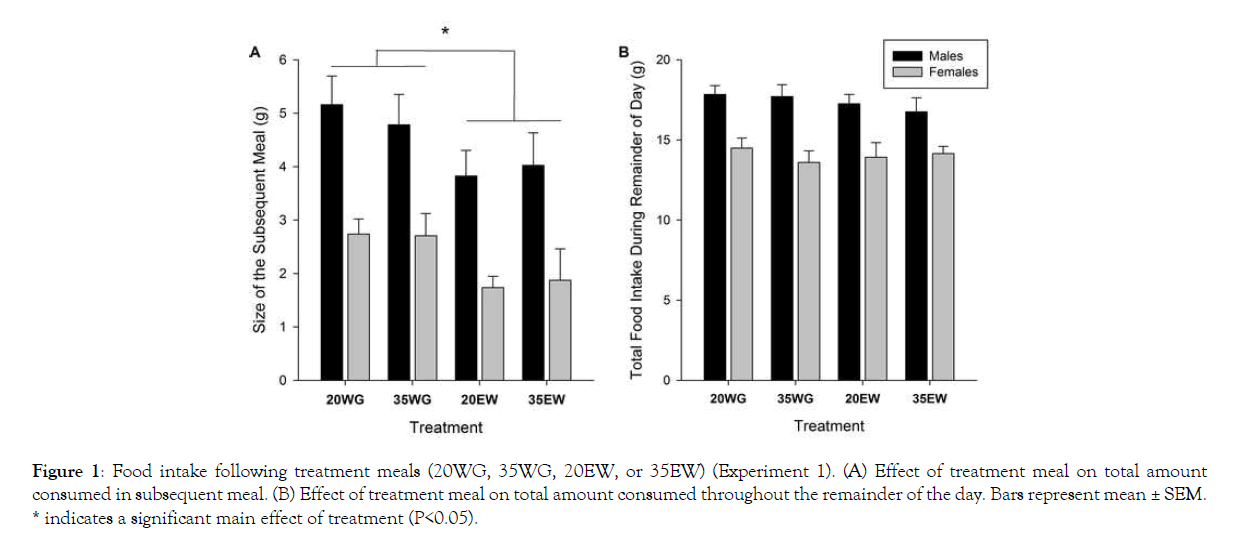
* indicates a significant main effect of treatment (P<0.05).
Figure 1: Food intake following treatment meals (20WG, 35WG, 20EW, or 35EW) (Experiment 1). (A) Effect of treatment meal on total amount consumed in subsequent meal. (B) Effect of treatment meal on total amount consumed throughout the remainder of the day. Bars represent mean ± SEM.
Collapsed across treatment groups, the respiratory exchange ratio (RER) increased following the test meal and stayed elevated until the start of the light cycle at 20:00 h (main effect of time, F23, 506=624.20, P<0.001; Figures 2A and 2B). The diets affected RER within the first 4 hours following the treatment meal, hence separate 2-way ANOVA analyses were conducted for only these time points. Results indicated lower RER for 35% than 20% protein diets for the first 4 hours following test meal ingestion. In addition, collapsed across level, egg white was lower than wheat gluten from 10:00 h through 12:00 h. No differences in RER were detected between sexes. As expected, energy expenditure was lower in females than males (F1, 22=54.45, P<0.001), but no significant differences between diet treatment groups were detected (Figures 2C and 2D).
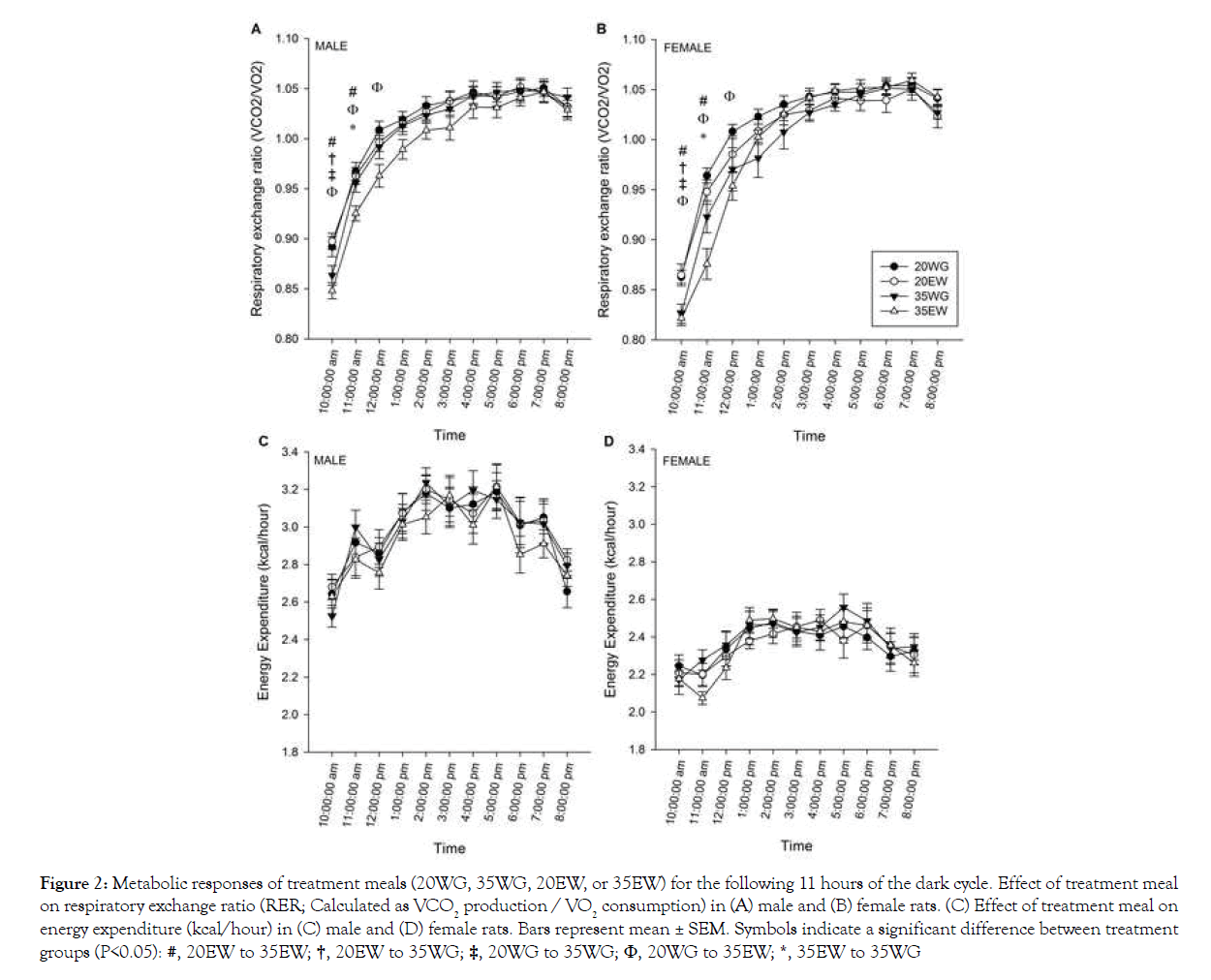
#, 20EW to 35EW; †, 20EW to 35WG; ‡, 20WG to 35WG; Φ, 20WG to 35EW; *, 35EW to 35WG
Figure 2: Metabolic responses of treatment meals (20WG, 35WG, 20EW, or 35EW) for the following 11 hours of the dark cycle. Effect of treatment meal on respiratory exchange ratio (RER; Calculated as VCO2 production / VO2 consumption) in (A) male and (B) female rats. (C) Effect of treatment meal on energy expenditure (kcal/hour) in (C) male and (D) female rats. Bars represent mean ± SEM. Symbols indicate a significant difference between treatment groups (P<0.05):
Experiment 2: Postprandial plasma amino acid and insulin
Amino acids: A total of ten amino acids were measured, seven of which are considered indispensable amino acids (lysine, methionine, leucine, isoleucine, valine, phenylalanine, tryptophan) and three non-essential amino acids (cysteine, alanine, and tyrosine). Significant effects of time indicated that all of the measured amino acids increased from baseline collapsed across treatment groups (all P <0.05), with the exception of methionine and tryptophan. Analysis of the sum of these ten measured amino acids (Figure 3A) revealed significant differences between treatment groups (F3, 12=11.53, P<0.001), but no significant interaction between treatment group and time point (F15, 54=1.39, P=0.1841). Post hoc analysis revealed increased total amino acids following 35EW compared to 35WG (P=0.029), and 20EW compared to 20WG (P=0.004). Two-way ANOVA collapsed across time points showed a main effect of protein type (F1, 12=15.19, P=0.002) indicating 37% increased overall amino acids in egg white treatments compared to wheat gluten treatments. Concentration of protein was not significant (F1, 12=1.24, P=0.287).
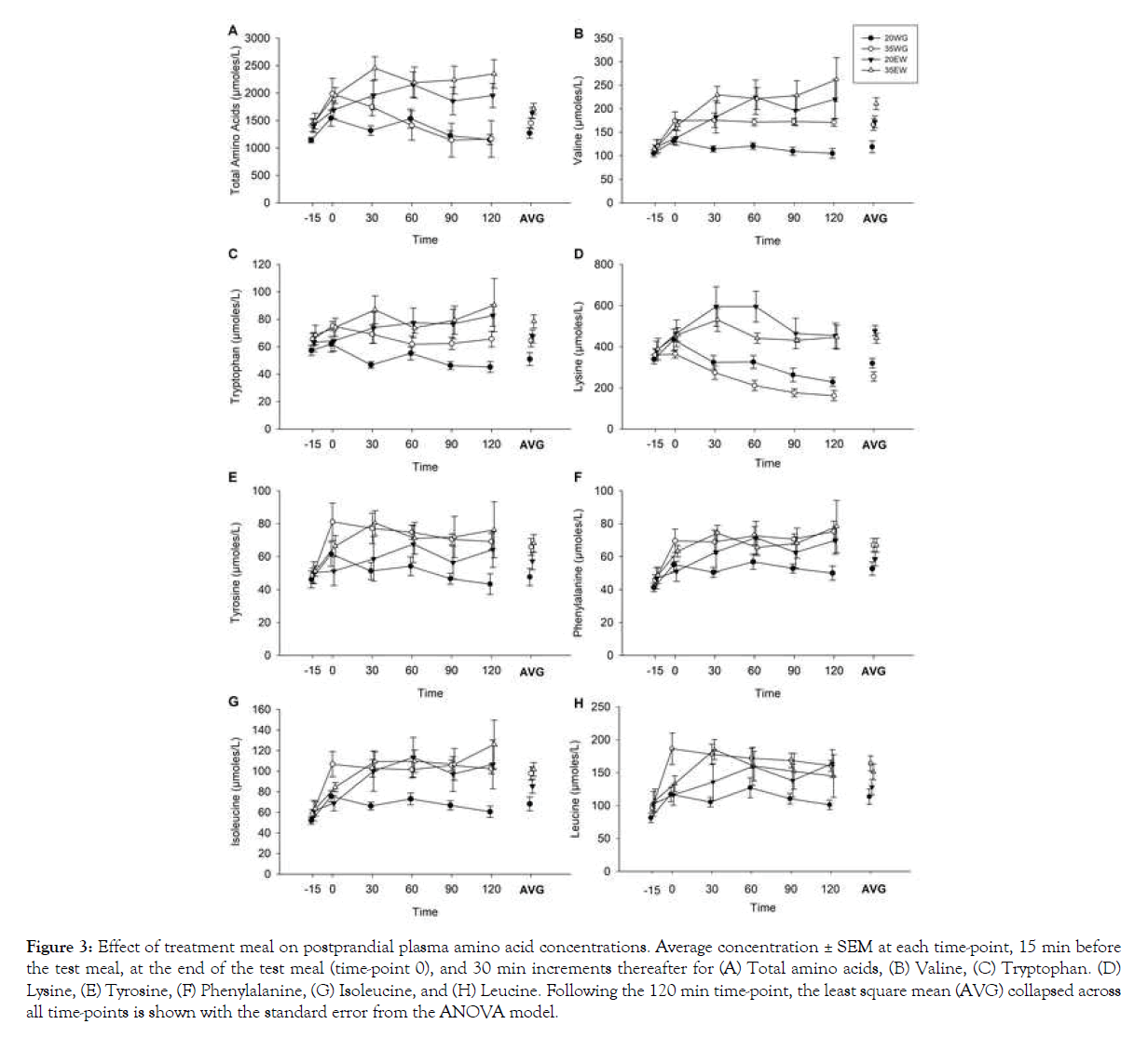
Figure 3: Effect of treatment meal on postprandial plasma amino acid concentrations. Average concentration ± SEM at each time-point, 15 min before the test meal, at the end of the test meal (time-point 0), and 30 min increments thereafter for (A) Total amino acids, (B) Valine, (C) Tryptophan. (D) Lysine, (E) Tyrosine, (F) Phenylalanine, (G) Isoleucine, and (H) Leucine. Following the 120 min time-point, the least square mean (AVG) collapsed across all time-points is shown with the standard error from the ANOVA model.
Considering each amino acid separately, significant differences between treatment groups were detected for valine (F3, 12=27.00, P<0.001; Figure 3B), tryptophan (F3, 12=17.56, P<0.001; Figure 3C), lysine (F3, 12=31.44, P<0.001; Figure 3D), tyrosine (F3, 12=11.78, P<0.001; Figure 3E), phenylalanine (F3, 12=8.04, P<0.005; Figure 3F), isoleucine (F3, 12=14.77, P<0.001; Figure 3G), and leucine (F3, 12=14.22, P<0.001; Figure 3H). No significant differences were observed for alanine and cysteine. Post hoc analyses of means collapsed across time revealed that egg white protein caused a greater postprandial increase in amino acids than wheat gluten at both 20% and 35% protein levels. Compared to 20WG and 35WG, 20EW and 35EW displayed increased lysine (both P<0.001), valine (P=0.001; P=0.004), tryptophan (P=0.005; P=0.015) and isoleucine (P=0.047; NS), respectively.
As expected, the two higher protein meals, 35EW and 35WG, showed increased postprandial concentrations of several amino acids compared to 20WG. This was indicated by post hoc analysis of treatments collapsed across time showing lower concentration following 20WG when compared to 35EW and 35WG for tyrosine (P=0.001 and P=0.002), isoleucine (P<0.001 and P<0.001), valine (P<0.001 and P=0.001), phenylalanine (P=0.007 and P=0.004), leucine (P=0.006 and P<0.001), and tryptophan (P=0.001 and P=0.002).
Interactions between treatment group and time point were detected for lysine (F15, 54=2.88, P=0.002), isoleucine (F15, 53=1.89, P=0.045), and valine (F15, 54=3.05, P=0.001). In the case of valine and isoleucine, the interaction effect was the result of valine and isoleucine in the 20WG group remaining close to baseline levels for the two hours during which blood samples were collected compared to the other treatment groups which increased from baseline (Figures 3B and 3G). Post hoc comparisons indicated isoleucine and valine following 20WG did not differ from baseline relative at any time points, whereas levels did differ from baseline following 20EW, 35WG, and 35EW (all P<0.01). The interaction effect for lysine was the result of decreasing lysine following wheat gluten treatments and increasing lysine following egg white treatments when compared to baseline values (Figure 3D). Post hoc comparisons indicated lysine following egg white increased from baseline at time point 30 and 60 min (both p-values <0.01), whereas levels decreased from baseline following wheat gluten at 60, 90, and 120 min (all P <0.05). Lysine and valine peaked immediately after ingestion of wheat gluten meals. However, egg white meals caused slower appearance of amino acids, with lysine peaking at 30 min and valine remaining elevated through the sampling period.
Two-way ANOVA collapsed across time points showed that egg white increased valine by 34% (F1, 12=16.13, P=0.002), tryptophan by 24% (F1, 12=9.41, P=0.010), and lysine by 60% (F1, 12=9.41, P=0.001), (Figures 3B, 3C and 3D). Main effects of protein concentration were detected for valine (F1, 12=10.48, P=0.007), tryptophan (F1, 12=7.46, P=0.018), tyrosine (F1, 12=7.05, P=0.021), phenylalanine (F1, 12=5.95, P=0.031), and isoleucine (F1, 12=9.42, P=0.009) in the expected direction with greater amino acids following meals composed of 35% protein as compared to 20% (Figures 3B, 3C, 3E, 3F, 3G). Interactions between protein type (egg white vs. wheat gluten) and concentration (20% vs. 35%) were not significant.
Insulin
Insulin levels increased following the meals, collapsed across treatment groups, as indicated by a significant effect of time in the repeated measures ANOVA (F5, 41=6.62, P<0.0001; Figure 4).
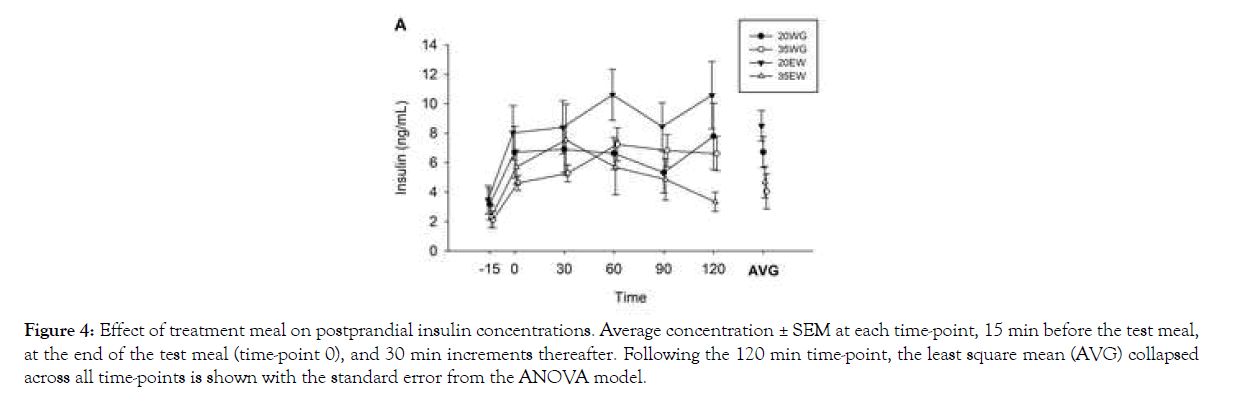
Figure 4: Effect of treatment meal on postprandial insulin concentrations. Average concentration ± SEM at each time-point, 15 min before the test meal, at the end of the test meal (time-point 0), and 30 min increments thereafter. Following the 120 min time-point, the least square mean (AVG) collapsed across all time-points is shown with the standard error from the ANOVA model.
Post hoc analysis indicated all time points following the meal (0- 120 min), collapsed across treatment groups, were different from baseline (all P <0.05). A main effect of treatment was also observed (F3, 10=8.37, P=0.004), but no significant interaction between treatment and time point (F15, 42=0.85, P=0.619) was detected. Post hoc analysis of treatments collapsed across time showed plasma insulin concentrations were greater following 20EW than 35WG (P=0.007) and 35EW (P=0.010). In the 2-way ANOVA where insulin measurements were collapsed across all time points, a main effect of protein concentration was observed (F1, 11=6.37, P=0.028; Figure 4). The 20% level displayed approximately 41% higher insulin levels than 35% protein. Type of protein was not significant (F1, 11=1.28, P=0.281). Neither was the interaction between type and concentration.
Experiment 3: Chronic diet intervention
Although rats in all four treatment groups displayed increased body weight over the course of the study (F2, 40=153.45, P<0.001), no significant differences were observed between treatment groups (Figure 5A). Neither were changes in fat mass, lean mass or the ratio of fat to lean mass between treatment groups detected (Figures 5B-5D). Daily food intake was not significantly different between treatment groups.
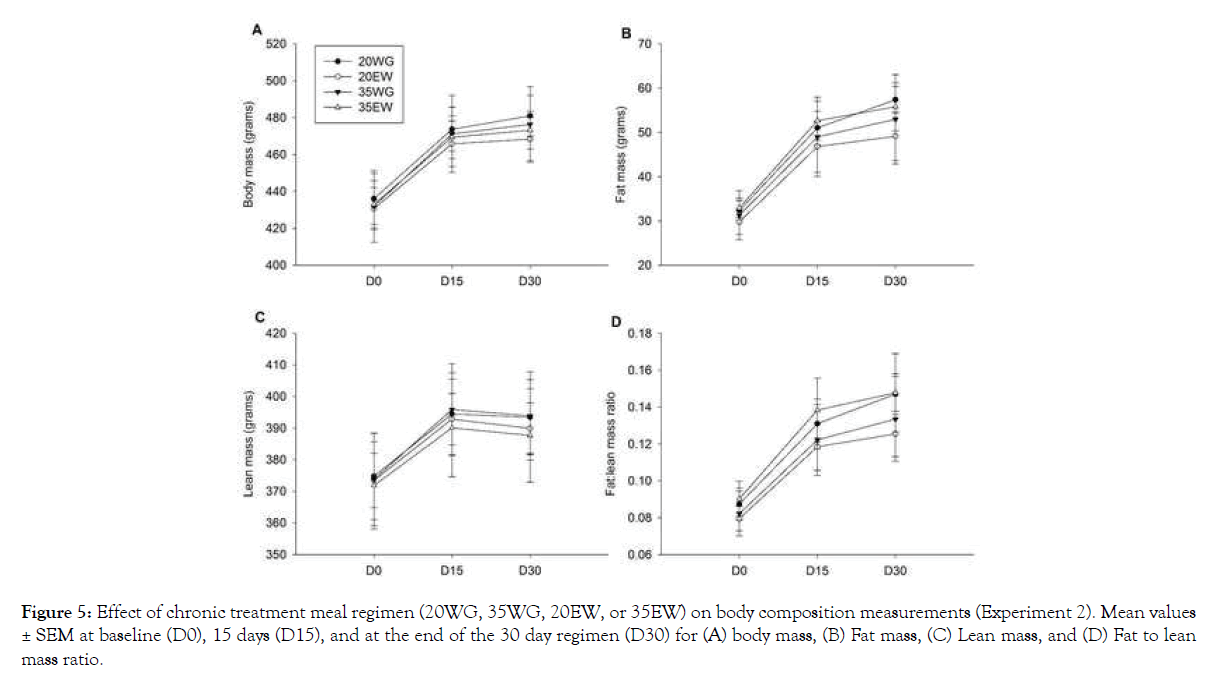
Figure 5: Effect of chronic treatment meal regimen (20WG, 35WG, 20EW, or 35EW) on body composition measurements (Experiment 2). Mean values ± SEM at baseline (D0), 15 days (D15), and at the end of the 30 day regimen (D30) for (A) body mass, (B) Fat mass, (C) Lean mass, and (D) Fat to lean mass ratio.
Overall RER decreased from the initial to final days of being of the diet regimen as indicated by a significant difference between baseline and final day (F1, 20=167.03, P<0.001). RER changed over the course of the day as reflected by a significant effect of time (F21, 420=143.45, P<0.001) (Figures 6A and 6B). An interaction between time and treatment was observed for RER during both the initial (F21, 420=453.15, P<0.001) and final (F21, 420=650.02, P<0.001) days of being on the diet regimen (Figures 6A and 6B). Results indicated the treatment differences were apparent for the first three hours following the treatment meal, hence separate 2 way ANOVA analyses were conducted for only these time points. Results indicated lower RER for 35% than 20% protein levels for the first 2 hours (F1, 44=6.60, P=0.010 for hour 1 and F1, 44=7.19, P=0.008 hour 2) during the initial day and four hours on the last day (F1, 44=14.38, P<0.001 for hour 1, F1, 44=30.07, P<0.001 hour 2, F1, 44=7.24, P=0.010 hour 3, F1, 44=5.03, P<0.05). In addition, collapsed across concentration, egg white was lower than wheat gluten for the first two hours during both initial and final days. Overall energy expenditure decreased by 2% over 30- day intervention period as indicated by comparing total energy expenditure at baseline vs. the final day (F1, 20=8.25, P=0.009) (Figures 6C and 6D). Energy expenditure significantly changed across the day, with higher expenditure during the night than daytime (F1, 20=2145.89, P<0.001). No significant differences of energy expenditure were detected between diet treatment groups (Figures 6C and 6D).
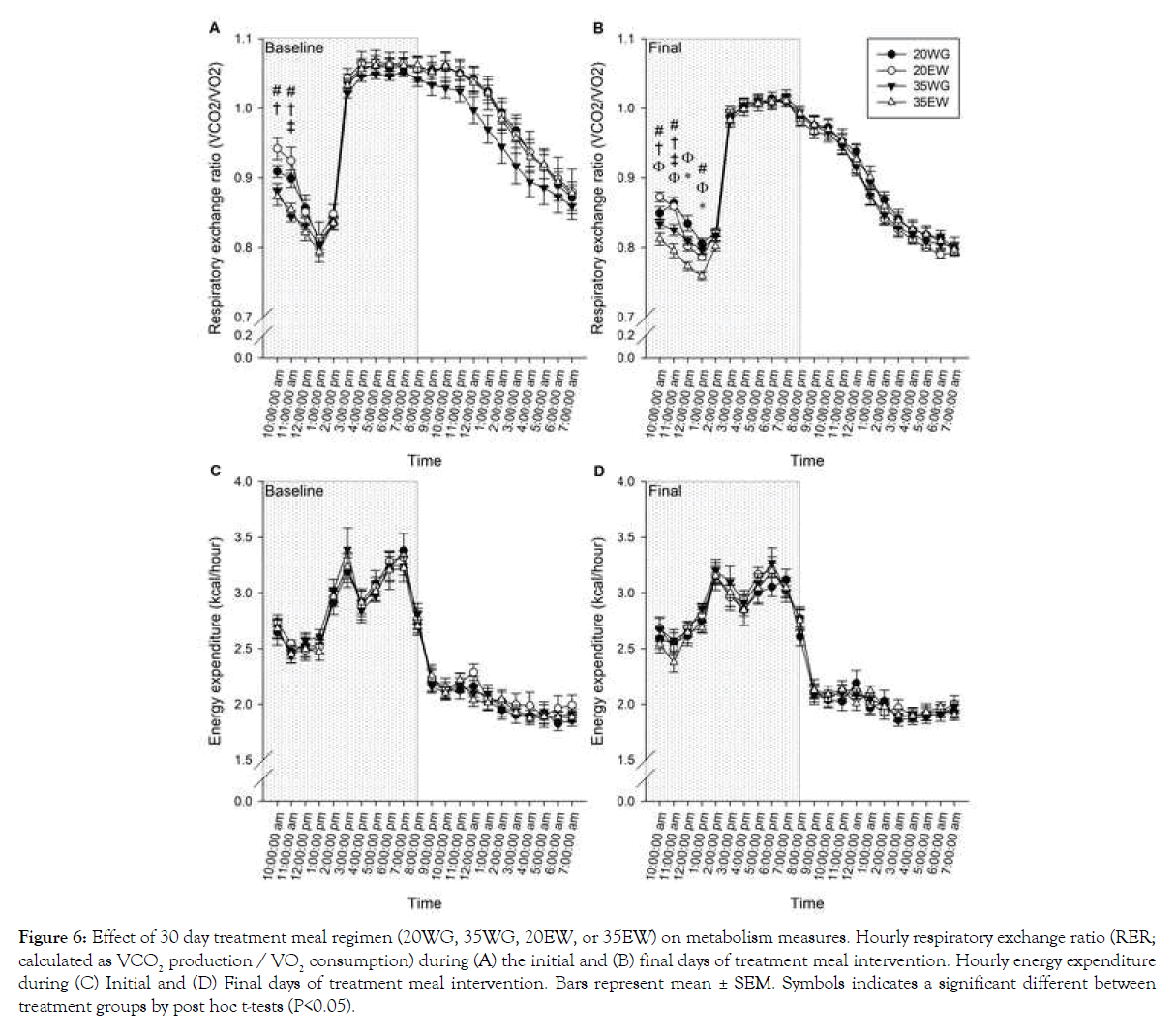
Figure 6: Effect of 30 day treatment meal regimen (20WG, 35WG, 20EW, or 35EW) on metabolism measures. Hourly respiratory exchange ratio (RER; calculated as VCO2 production / VO2 consumption) during (A) the initial and (B) final days of treatment meal intervention. Hourly energy expenditure during (C) Initial and (D) Final days of treatment meal intervention. Bars represent mean ± SEM. Symbols indicates a significant different between treatment groups by post hoc t-tests (P<0.05).
In the present study we repeated a previous finding that meals containing egg white protein induce greater satiety than wheat gluten protein [14] and have further extended this finding to both male and female rats and for both 35% and 20% protein levels. We further showed that the increased satiety from egg white was accompanied by increased postprandial plasma amino acids and lowered respiratory exchange ratio (RER).
Although we observed that satiety differs between protein sources, no significant differences were detected between the levels of protein (20% vs. 35%) (Figure 1A). While the concentrations of protein used in this study are within the range typically consumed in high protein diets, it is possible that both concentrations surpassed the minimum threshold levels required to trigger protein-induced satiety [43] resulting in equivalent effects on satiety measures (non-detectable differences between levels). The concentrations of protein tested in this study (20 and 35%) were both greater than the standard recommended average amount of protein (12- 18% protein) in rodent and human diets. These percentages were selected because they reflect the 90th percentile of protein intake (20% protein) and a more typically recommended level in high protein diets (35% protein) [40].
In addition, to establishing short-term differential satiety-inducing effects from egg white vs. wheat gluten, this study also explored potential mechanisms for the enhanced satiety-inducing effect of egg white protein. Egg white protein resulted in greater total levels of overall plasma amino acids (Figure 3A), but also in a distinct profile of amino acids when compared with wheat gluten meals (Figures 3B-3G). Specifically, we observed that egg white protein resulted in higher plasma levels of lysine, valine, and tryptophan in comparison to wheat gluten protein (Figures 3B-3D), which have been identified in previous studies to have anorexigenic effects [7,9,44,45]. In particular, valine, a branched chain amino acid, has been found in other studies to be associated with reduced subsequent food intake and increased satiety ratings [21,46-48]. The increased valine availability following egg white protein meal in the present study may result in a more efficient transport across the blood-brain barrier in comparison to other large neutral amino acids [49]. Increased valine availability in the brain may signal energy availability, which might contribute to the differences in satiety observed in the present study. Among the three branched chain amino acids, valine is the only one to be solely gluconeogenic and as such, able to contribute to the glucose pool [50]. Glucose levels are sensed and regulated in hypothalamic glucose sensing neurons, which activate pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus to decrease food intake [51,52]. While central administration of valine effects on gluconeogenic activity and subsequent food intake response has not been directly investigated, its possible involvement in protein-induced satiety warrants further consideration. On the other hand and despite being one of the most studied amino acids in association with satiety signaling effects, [53,54] leucine levels do not seem to explain the observed differences in satiety following egg white and wheat gluten in the present study (Figure 3H). Taken together, these results suggest that the satiety enhancing effects of egg white protein may be partially related to their ability to increase overall plasma amino acid levels, and/or increases in specific amino acids, such as valine.
Contrary to our hypothesis, no differences in insulin levels were detected between egg white and wheat gluten protein at both the 20% and 35% protein levels following the meals (Figure 4). This suggests that circulating insulin levels are unlikely to explain the differences in satiety effects observed between egg white and wheat gluten. As expected, the insulin response reflected the treatment meal’s carbohydrate content, with greater insulin levels detected following the 20% protein meal in relation to 35% protein meal. Owing to the limited blood samples obtained, we were unable to measure glucose levels but given greater carbohydrate contribution of the diet in the 20% protein groups (see Methods – Experimental diet preparation above), we would expect glucose levels would be higher in 20% than 35% protein meal.
While insulin levels are not affected by the protein source ingested, other satiety hormones released as a result of protein ingestion may be involved in satiety differences among protein sources. These satiety inducing hormones include cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), and peptide YY (PYY). Evidence suggests that amino acids and oligopeptides released during dietary protein digestion cause release of these satiety hormones to transmit information about energy intake to the central nervous system [23-25]. Additionally, ghrelin release from the stomach is inhibited in the presence of amino acids in the gastrointestinal tract [26] and therefore decreased ghrelin could also be involved in the satiety signalling. Future studies should consider assessing levels of CCK, GLP-1, PYY, and ghrelin in response to ingestion of different protein sources for interpreting satiety outcomes.
Increased diet-induced thermogenesis has been previously suggested to be involved in protein-induced satiety [39,55] and was therefore hypothesized to be involved in the differences in satiety detected in the present study. However, our data indicate similar energy expenditure following ingestion of the different protein sources. While some studies report differences in energy expenditure following ingestion of meals containing different protein sources, [55,56] other studies have found no differences, for example between whey and casein [6,57]. It is possible that protein sources may only be causing differential energy expenditure at much higher proportions of dietary protein intake, as these studies provided subjects with 50-60% protein [55,56]. On the other hand, clinical studies have further shown increased subsequent and 24-hour energy expenditure following high protein diets, when compared to low protein control diets [37] suggesting that level of protein alone can impact energy expenditure. However, even in such studies, differences in energy expenditure between protein treatment groups were modest [37]. As such, it is possible that the levels of protein used in the present study were too similar (20% and 35%) to be able to induce detectable differences in energy expenditure due to level of protein, as previous studies suggest. Whilst we did not detect differences in energy expenditure between treatment groups, 35% egg white protein caused lower respiratory exchange ratio (RER) during the first several hours, indicating increased protein metabolism. Taken together, these results suggest that energy expenditure probably does not play a large role in the differential satiety resulting from the consumption of egg white vs. wheat gluten.
Overall, meals containing egg white protein decreased subsequent food intake and, in the case of 35% egg white protein, lowered respiratory exchange ratio relative to wheat gluten (Figures 1A, 2A and 2B). However, the satiety and metabolic responses were short-lived, lasting for only up to 4 hours following meal ingestion. Furthermore, the manipulation of the protein source at the first meal of the day for a 30-day period had minimal long-term effects on body weight and composition (Figure 4). Given the short-lived metabolic and satiety effects of high protein meals, changes in body weight and composition will likely require continuous consumption of such protein meals throughout the day. Future studies should focus on long-term feeding of such different protein sources throughout the day to evaluate whether under these conditions; egg white protein may be effective at reducing overall food intake, body weight and fat deposition. Furthermore, this study adds further evidence to previous findings demonstrating that ingestion of isoenergetic amounts of protein from different protein sources can cause unique patterns of circulating plasma amino acids, and that differences in satiety between protein sources are likely related to the resulting differences in amino acid availability [7,9,58-60]. To confirm causality in the direction of the relationship, future studies should explore the extent to which administration of these amino acids directly into the circulation at physiologically relevant levels result in acute decreases in food intake.
The present work illustrates the importance of considering protein source when designing high-protein diets to control appetite. Our work shows that egg white protein may be more satiating than wheat gluten, and therefore should be considered when formulating high-protein diets for weight management. Given the obesity epidemic, and the large number of subjects in the United States and around the world that are incorporating a high-protein diet into their life in order to manage weight gain, it is crucial that we establish the fundamental knowledge about the satiety inducing effects of specific protein sources and resulting post-prandial amino acid profiles. Such knowledge will allow us to make evidence-based decisions about diet and health moving forward.
This study was funded from indirect costs recovered (ICR) from federal grant DK082609 and ICR from various donors.
The authors have declared that no competing interests exist.
Citation: Du K, Markus E, Fecych M, Lee Beverly J, Rhodes JS, Rendeiro C (2019) Metabolic Consequences of Egg White versus Wheat Gluten Protein Consumption in a Rodent Model. J Nutr Food Sci 9:761.
Received: 10-May-2019 Accepted: 18-Jun-2019 Published: 25-Jun-2019 , DOI: 10.35248/2155-9600.19.9.1000761
Copyright: © 2019 Du K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This study was funded from indirect costs recovered (ICR) from federal grant DK082609 and ICR from various donors.