Research Article - (2017) Volume 3, Issue 2
Microbiological and Physicochemical Qualities of Selected Commercially Produced Poultry Feeds Sold In Umudike, Abia State, Nigeria
*Corresponding Author: Ukaegbu-Obi KM, Department of Microbiology, College of Natural Sciences, Michael Okpara University of Agriculture Umudike, PMB 7267 Umuahia, Abia State, Nigeria, Tel: 0002-9402-9263 Email: ,
Abstract
Four different commercial poultry feeds namely, Amo, Vital, Top and Animal care poultry feed brands, each consisting of starter, grower, layer and finisher categories respectively, obtained from their trade outlets in Umudike, Nigeria were examined for their microbiological and physicochemical qualities using standard microbiological and analytical methods. The fungal count was highest in Vital layer feed with 9.6 × 105 cfu/g, while the least count of 3.0 × 104 cfu/g was recorded in Animal care grower feed. Bacterial count was highest in Amo starter (2.70 × 107 cfu/g) and least in Amo layer (2.0 × 106 cfu/g). A total of twelve microorganisms were isolated. Fungal isolates include Aspergillus niger, Aspergillus flavus, Fusarium spp, Penicillium spp and Rhizopus spp with Aspergillus flavus having the highest percentage occurrence of 25.4%. Bacterial isolates include Staphylococcus aureus, Escherichia coli, Salmonella spp, Pseudomonas spp, Klebsiella spp, Streptococcus spp, and Listeria spp with Staphylococcus aureus having the highest percentage occurrence of 19.9%. Physicochemical analyses revealed the presence of moisture, ash, carbohydrate, crude fibre, crude fat and protein contents. The presence of microorganisms in poultry feeds could pose a threat to the poultry birds and humans, therefore the need for constant assessment of the safe quality of these feeds.
Keywords: Poultry feeds; Microbiological; Physicochemical; Assessment; Commercial
Introduction
There is an increase demand for poultry at all times of the year as poultry provides economically profitable resources of high quality protein for human consumption. Poultry farming serves as a major source of income as well and provides job [1].
In an effort to achieve rapid bird growth to meet the increasing demand for birds and more profit by poultry farmers, large quantities of nitrogenous waste fortified with other supplements such as spent grain, cassava waste, bone meal are compounded as poultry feed [2,3]. Thus, there are various categories of feeds depending on what they are destined to achieved in poultry birds e.g. starter, grower, Layer and finisher category of feeds.
Poultry feed is considered as one of the important sources of contamination to poultry [4] as they are routinely subject to contamination from diverse sources including environmental pollution, activities of insects and microbes [5]. Specifically, some of the additives have been incriminated amongst the principal sources of bacteria of public health concern [3,6].
Standard poultry feeds possess considerable percentage of ash, crude fat, crude fibre protein, moisture and carbohydrate contents which are vital for the poultry animal’s health and development, these nutrients may as matter of fact form nutrients for utilization by contaminating microorganisms [7]. Both pre-harvest and post-harvest biological contaminants can be transmitted through feed ingredients to the mixed feed and finally to poultry animals [8].
Different types of farm animal diseases such as dysentery, fowl cholera, samonellosis, staphylococcosis. colibacillosis, erysipelas, listeriosis have been associated with poultry feed [9]. Microorganisms that can contaminate poultry feeds includes Escherichia coli , Staphylococcus aureus , Salmonella spp, Listeria spp, Streptococcus spp, Klebsiella spp, Pseudomonas spp, Aspergillus niger, Aspergillus flavus, Rhizopus spp, Penicillium spp, Fusarium spp. However, the number and types of microorganisms in poultry feeds vary depending on the function of materials, location of its origin, climate conditions encountered, harvesting, processing, storage, transport technologies employed and packaging materials [10].
Effects of microorganisms in poultry feeds may include degradation of nutrient value, change in smell and colour, caking of the feed and production of toxins [11], example of these toxins include mycotoxins which differ in their toxicological effect and are usually found in mixed form [12]. The occurrence of mycotoxigenic fungi is widespread in tropical countries due to favourable environmental conditions [13,14]. Unsafe poultry feed may also lead to great economic losses in case of destroying an infected flock of birds [15]. As it has become a popular industry for small scale holders and this has contributed sparingly to the economy of these countries [11].
The quality of poultry feed is of public health importance because it affects the quality of poultry and the wholesomeness of meat consumed by man. The safety and quality of these poultry feeds are currently of major concern in developed countries. Safety of feed is a fundamental requirement for all birds. Considering the health hazard posed to poultry birds and the unsuspecting consumers of such contaminated feeds and its overwhelming socioeconomic impact, it is essential to undertake this study
This study was therefore carried out to isolate and characterize the microorganisms associated with different brands of poultry feeds in Umuahia associated with different brands of poultry feeds in Umuahia. Also to determine the physicochemical properties of these commercially produced feeds.
Materials and Methods
Sample collection
Four different brands of poultry feeds namely; Top, Vital, Amo and Animal care feeds each consisting of starter, grower, layer and finisher feed types respectively, were purchased from the retailers in Umuahia market, ensuring that each had no abnormal colour change or odour. The samples were collected aseptically into a sterile plastic bag and labelled accordingly; they were transported immediately to the laboratory for analyses.
Sample preparation
One gram of each of the collected sample was weighed out for microbiological analyses using a triple beam balance (Model: OHAUS 750-S0). For each of the feed samples obtained, a ten-fold serial dilution was carried out using physiological saline, these dilutions was made up to 10-7.
Microbiological analyses
Isolation and enumeration of fungal isolates: An aliquot of the dilution was cultured by spread plate technique using Sabouraud dextrose Agar. The plates were incubated in duplicates for 24-72 hours; after which colonies on the plates were counted, recorded and the mean fungal counts expressed in colony forming unit per gram (cfu/g) of each sample.
Characterization and identification of fungal isolates: The identification and characterization of fungal isolates were based on morphological features, slide culture technique and slide mount of each isolate in lactophenol-cotton blue as described by Barnett and Hunter [16].
Isolation and enumeration of bacterial isolates: One gram of each sample was added into 9 ml physiological saline and serially diluted up to 10-7. An aliquot was cultured by spread plate technique onto Nutrient Agar, McConkey Agar and Blood Agar in duplicates. The plates were incubated at 37°C for 24 hours. Colonies of bacteria that grew after incubation were counted, recorded and the mean bacteria count of the duplicate plates expressed as colony forming unit per gram of the sample.
Characterization and identification of bacterial isolates: The discrete bacteria colonies that grew were sub-cultured on a freshly prepared Nutrient Agar to obtain pure cultures. The pure cultures were maintained at 4°C for further biochemical tests.
Physicochemical analyses
Determination of moisture content (Mc): The method of Pearson [17] was used. The weight of an empty aluminium dish Can (W0) was determined before the sample was introduced into it, about Twenty (20) grams of the feed sample was measured out and further weighed (W1) in the aluminium dish Can. The aluminium dish can was then dried in a hot air oven for 24 hours and cooled in a desiccators and weighed (W2) measured. Percentage of moisture content was determined as follows:
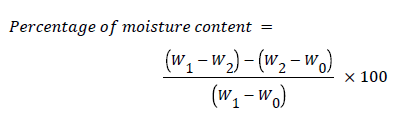
Where,
W0=Weight of empty moisture can
W1=Weight of moisture can and sample
W2=Weight of dessicated sample
(W1-W0)-(W2-W0)=Weight loss
Determination of ash content (Ac): The method reported by AOAC [18] was used. An empty clean crucible dish was weighed noted as W0. Poultry feed sample in two (2) grams was measured and weighed in the empty clean crucible; the weight (W1) was noted. The crucible containing the sample was further placed in a muffle furnace and ashed at 5000°C–6000°C for about 8 hours the crucible was removed from the muffle furnace and cooled in a desiccator, and weighed (W2) using a weighing balance

Where,
W0=Weight of empty crucible
W1=Weight of sample and crucible
W2=Weight of ash and crucible
Determination of crude fibre content (Cfic): The method reported by AOAC [18] was used, Twenty (20) grams of feed sample were weighed in conical flask and the weight (W0) noted. 200 ml of boiling 1.25% H2S4 were added into the conical flask containing the sample and boiled gently for 30 minutes. The content was filtered through a muslin cloth and the residue scraped into a clean conical flask using a spatula 20 ml of boiling 1.25% of NaOH added and allowed to boil gently for 30 minutes. Further filtration was done using the Buckner pressurized filter through a muslin cloth spread over its funnel. The resulting residue was done using the Buckner pressurized filter through a muslin cloth spread over its funnel. The resulting residue was rinsed once with 10% HCl and twice with ethanol. Final rising with petroleum ether was done thrice and allowed to drain. The residue was scraped into a flat silica dish and dried overnight in oven at 105°C. Afterwards, it was cooled in desiccator and the sample weighed (W1). The sample was further ashed at 550°C for 90 minutes in a muffle furnace, cooled again in the desiccators and reweighed. Percentage fiber content was determined as follows:
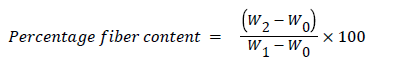
Where,
W0=Weight of sample
W1=Weight of insoluble material
Determination of crude protein content: The method reported by AOAC [18] was used. The feeds samples were measured in Five (5) grams in a measuring cylinder and introduced into 100 ml of micro kjeidahl digestion flask. The following were further added to the flask: 5 ml of distilled water, one tablet of mercury kjeidahl catalyst, half spatula full of K2SO4, 5 ml of concentrated H2SO4. The flask was placed on a 6-unit digester (model: 65500) inside the fume cupboard and digested for 3 hours, after the digestion was completed, the content was cooled; leaving behind a colourless residue. Some quantity of distilled water was added to the colourless digest and the flask swirled until everything was dissolved. The content was emptied into a 100 ml volumetric flask.
The malkam distillation apparatus was set up and nitrogen distillation carried out as follow; 5 ml of the extract were pipetted into the distillation pot, followed by 5 ml of 55% NaOH and distilled carried out for 15 minutes. During the distillation, a 100 ml/conical flask containing 5ml boric acid was kept under the condenser to collect the distillate boric acid was observed for any change in colour.
Determination of crude fat content: The method reported by AOAC [18] was used. A 250 ml round bottom flask was dried in an oven, allowed to cool in the desiccator and the weight measured. 200 ml of petroleum ether were introduced into dry 250 ml round bottom flask. Ten (10) grams of the samples were measured using a measuring cylinder and weighed in a labelled porous thimble, which was covered with a clean white cotton wool. The covered thimble was placed into the soxhlet extractor (Model: EME 60250/CE) fitted into the round bottomed flask. The extraction apparatus was set up with the flask on the hot water bath of the soxhlet extracting unit. The content of the round the ether soluble constitutes into the round-bottomed flask. The extraction lasted for 5-6 hours during which all the petroleum ether moved up to the extractor, leaving behind the fats in the round bottom flask. The porous thimble was removed with care and the petroleum ether collected from the top for re-use. The round-bottomed flask containing the lipids was then removed and oven-dried for 1 hour at 105-110°C. After drying it was further transferred into a desiccator (containing silica gel) where it was cooled and also weighed.

Where,
W0=Weight of empty porous thimble
W1=Weight of thimble and sample
W2=Weight of empty extraction flask
W3=Weight of extraction flask and extract
W1–W0=Weight of sample
W3–W2=Weight of extract
Determination of carbohydrate content: The method of Osborne and Voogt (1978) was used. Carbohydrate content was determined indirectly by subtracting the crude protein, crude fibre, crude lipid, ash moisture content from 100% as follows:
Percentage Carbohydrate Content (% CC)=100-(% protein+% Fibre +% Ash+% Moisture +% Fat).
Statistical analysis
The chi-square statistics at 5% probability level using SPSS (Statistical Package for social sciences, version 22.0) was employed to determine the significant difference between the observed and expected data.
Results
A total of 12 microorganisms were isolated. The bacterial genera were seven in number while the fungal isolates were five.
From the mean fungal counts of each feed sample, C2 (vital layer) had the highest fungal count 9.6 × 105 cfu/g while the least fungal count was recorded in B4 (Animal care grower) with 3.0 × 104 cfu/g (Table 1).
| Sample code | Fungal Counts (cfu/g) | Bacterial Counts (cfu/g) |
|---|---|---|
| 1A1 | 3.90 × 104 | 2.70 × 107 |
| B1 | 3.40 × 104 | 1.90 × 107 |
| C1 | 5.00 × 104 | 2.00 × 106 |
| D1 | 4.00 × 104 | 2.60 × 106 |
| 2A2 | 4.20 × 105 | 1.67 × 107 |
| B2 | 3.40 × 104 | 2.00 × 107 |
| C2 | 9.60 × 105 | 2.25 × 106 |
| D2 | 3.40 × 104 | 1.60 × 107 |
| 3A3 | 3.00 × 105 | 1.50 × 107 |
| B3 | 3.60 × 104 | 1.27 × 107 |
| C3 | 5.80 × 104 | 2.50 × 106 |
| D3 | 3.70 × 105 | 1.77 × 107 |
| 4A4 | 4.20 × 104 | 2.00 × 107 |
| B4 | 3.00 × 104 | 1.60× 107 |
| C4 | 5.60 × 105 | 2.70 × 106 |
| D4 | 3.10 × 105 | 2. 20 × 107 |
Table 1: Mean fungal and bacterial counts (cfu/g) of different poultry feed samples (1: Amo feed; 2: Vital feed; 3: Top feed; 4: Animal care. A=Starter; B=Grower; C=Layer; D=Finisher; +=present; -=absent).
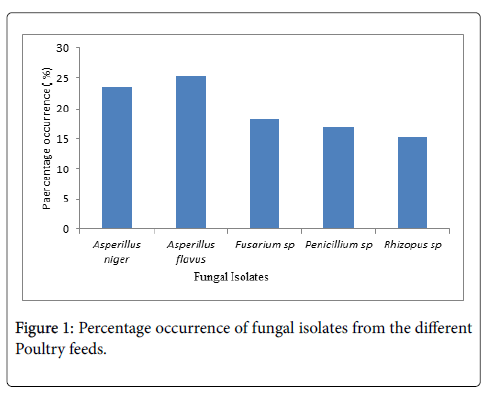
The occurrence of fungal isolates in various feed samples. The percentage occurrence of fungal isolates in various feed samples showed that Aspergillus flavus had the highest percentage occurrence (25.4%) (Figure 1).
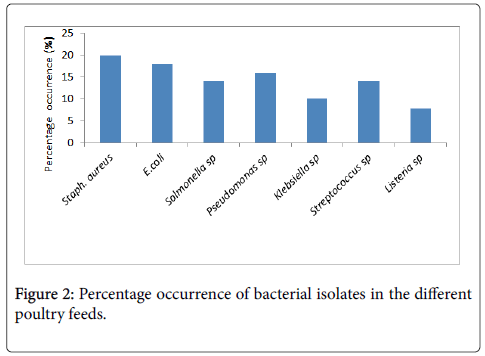
The mean bacterial count of each feed sample showed that A1 (Amo layer) had the highest count of 2.70 × 107 cfu/g and the least count was obtained from C1 (Amo layer) with 2.0 × 106 cfu/g (Figure 2). The percentage occurrence of bacterial isolates showed that Staphylococcus aureus had the highest occurrence of 19.9% (Table 2).
| Sample | Feed category | Aspergillus niger | Aspergillus flavus | Fusarium spp | Pencillium spp | Rhizopus spp |
|---|---|---|---|---|---|---|
| 1 | A | - | - | - | + | - |
| B | + | + | + | - | - | |
| C | + | + | + | + | + | |
| D | + | + | + | + | + | |
| 2 | A | + | + | + | + | + |
| B | - | + | + | + | - | |
| C | + | + | - | + | + | |
| D | + | + | + | + | + | |
| 3 | A | + | + | - | - | - |
| B | + | + | + | - | + | |
| C | + | + | - | - | + | |
| D | + | + | + | + | - | |
| 4 | A | + | + | + | - | - |
| B | + | + | + | + | - | |
| C | + | + | - | - | + | |
| D | + | + | + | + | + |
Table 2: Occurrence of fungal isolates from different brands of poultry feeds (1: Amo feed; 2: Vital feed; 3: Top feed; 4: Animal care. A=Starter; B=Grower; C=Layer; D=Finisher; +=present; -=Absent).
The physicochemical analyses of the feed samples, moisture content (MC) ranged from 4.80 to 13.20%, Ash content (AC) ranged from 7.50 to 18.00%, crude fat content (CFC) ranged from 3.40 to 10.00%, crude protein fiber (CPC) ranged from 4.80 to 30.00%, crude fiber content (CFiC) ranged from 4.00 to 34.00% and carbohydrate content (CC) was from 20 to 70% respectively (Tables 3 and 4).
| Samples | Feed category | S. aureus | E. coli | Salmonella spp | Pseudomonas spp | Klebsiella spp | Streptococcus spp | Listeria spp |
|---|---|---|---|---|---|---|---|---|
| 1 | A | + | + | + | - | - | + | - |
| B | - | - | - | - | - | + | - | |
| C | + | + | - | - | - | - | - | |
| D | + | - | - | + | - | + | + | |
| 2 | A | + | + | + | - | - | - | + |
| B | - | - | + | - | + | - | - | |
| C | + | + | - | + | - | + | - | |
| D | + | + | - | + | - | - | - | |
| 3 | A | + | - | + | - | + | + | - |
| B | - | - | - | + | - | - | - | |
| C | - | + | + | - | + | - | - | |
| D | + | + | + | + | - | + | + | |
| 4 | A | + | + | - | - | + | - | + |
| B | - | - | - | + | - | + | - | |
| C | - | + | + | + | - | - | - | |
| D | + | + | - | + | + | - | - |
Table 3: Occurrence of bacterial isolates from different brands of poultry feeds (1: Amo feed; 2: Vital feed; 3: Top feed; 4: Animal care. A=Starter; B=Grower; C=Layer; D=Finisher; +=present; -=Absent).
| MC% | AC% | CFC% | CPC% | CFiC% | CC% | |
|---|---|---|---|---|---|---|
| 1A | 5 | 12.7 | 6.8 | 16.5 | 34 | 25.8 |
| B | 4.8 | 7.5 | 10 | 9.7 | 8.5 | 60 |
| C | 7.6 | 12.5 | 3.4 | 9.8 | 16.7 | 50.2 |
| D | 8.5 | 8 | 7.4 | 13 | 5 | 88.1 |
| 2A | 8 | 15 | 6.3 | 17.2 | 5 | 50 |
| B | 10 | 15 | 9.2 | 30 | 13 | 24.6 |
| C | 9.2 | 18 | 4.8 | 8 | 6.2 | 54 |
| D | 7 | 16 | 8 | 7.6 | 4.8 | 58 |
| 3A | 7 | 14.6 | 4.2 | 25 | 4 | 46 |
| B | 7.8 | 12 | 7 | 20 | 3.8 | 50 |
| C | 8 | 10 | 7.2 | 26 | 4 | 45 |
| D | 7.4 | 10.7 | 5.3 | 30 | 4.8 | 40 |
| 4A | 5 | 8.7 | 9.5 | 5 | 4 | 68 |
| B | 8.7 | 15 | 6 | 4.8 | 5 | 60.5 |
| C | 13.2 | 15 | 7 | 25 | 22 | 20 |
| D | 7 | 9.2 | 7.4 | 4 | 4 | 70 |
Table 4: Physicochemical analyses of poultry feed samples (Key: 1: Amo feed; 2: Vital feed; 3: Top feed; 4: Animal care. A=Starter; B=Grower; C=Layer; D=Finisher; MC: Moisture content; AC: Ash content; CFC: Crude fat content; CPC: Crude protein content; CFiC: Crude fiber content; CC: Carbohydrate Content).
Discussion
All poultry feed samples examined showed the presence of microorganisms. When isolated and identified, the fungi isolated included Aspergillus niger, Aspergillus flavus, Fusarium spp, Penicillium spp, Rhizopus spp, while the bacterial genera were Escherichia coli, Klebsiella spp, Listeria spp, Pseudomonas spp, Salmonella spp, Staphylococcus aureus and Streptococcus spp. Some of these microbial isolates have also been isolated by Sule and Ilori [19], Uwaezuoke and Ogbulie [3]. The presence of these microorganisms in the poultry feed samples is of public health concern as these microorganisms can be pathogenic causing infections to the birds and also to the consumers of the poultry birds. Microbial presence in these poultry feeds shows that these microbes can utilize these feeds for their growth and metabolism.
Sources of these organisms may be of various origins. As reported by D’Mello [10] microbial contamination of poultry feeds can be as a result of climatic conditions encountered during processing, storage and transport stages and technologies employed. The bacterial genera may have originated from nitrogenous waste such as dung, chicken excreta, etc. as reported by Uwaezuoke and Ogbulie [3], fungal species may have resulted from carry-over of over-seasoned fungal species from the field as fungal species have the ability to transform into spores that can remain dormant for very long time [7].
The observed high mean bacterial counts in starter and finisher category of feed samples agree with the findings of Arotupin et al., [7]. This can be as a result of the ingredients used in producing these categories of feeds.
The observed high occurrence of fungal species agrees with reports of the experiment conducted elsewhere by Uwaezuoke and Ogbulie [3]. This high level of fungi obtained in this study can be associated with the low water activity of poultry feed and the physiology of the contaminating fungal genera, moisture content and ambient temperature. These are key factors affecting fungal colonization of poultry feeds and mycotoxin production in concentrate and compounded feeds. Aspergillus and Penicillium are involved in production of Aflatoxins which are powerful mycotoxins that has been documented to be carcinogenic to human [20]. Fusarium spp and Rhizopus spp should be viewed with serious concern, more than not these organisms have been documented to be the most dominant of all the fungi in respect of mycotoxin production in poultry feeds [5].
The greater number of bacteria than fungi isolated agrees with the findings of Uwaezuoke and Ogbulie [3] and Obi and Ozugbo [21]. But not in consonance with the findings of Arotupin et al., [7] which had higher number of fungi than bacteria in poultry feed assessment.
The presence of Listeria, Pseudomonas, Escherichia coli, Klebsiella and Salmonella may suggest faecal as well as environmental contamination. Some of these organisms are well known pathogens of birds and farm animals. For example even a small percentage of listeria spp may be significant as reported by Aliyu et al., [11]. Staphylococcus aureus, a normal flora of skin and nose portrays improper handling practices. The use of animal protein ingredient especially cheap locally processed fish waste has been reported to be an important vehicle for bacterial contamination in poultry feed ingredient [7].
The association between the feed types and mean bacteria counts was not statistically significant, X2=0.75, p>0.05.
The physicochemical analyses show that the various poultry feeds constitutes of nutrients that support and sustain microbial growth.
The presence, load and percentage of these isolated bacteria and fungi in these feeds under study cause degradation of feed nutrients, production of toxins and proliferation of microorganisms. These affect the quality of the poultry feeds, birds and bring about losses to farmers as result of death of poultry birds. The wholesomeness of poultry meat consumed by man is also affected which leads to food borne illnesses.
An addition of an antimicrobial substance which will inhibit microbial growth can be added poultry feed. Production processes should be aseptically carried out to avoid microbial contamination. These feeds should be properly stored in a dry place while prolonged storage should be avoided. Regular safe quality and accurate assessment of these feeds is essential considering the possible socio-economic and health implications of contaminated poultry feeds to poultry and human consumers of poultry products.
References
- Ezekiel CN, Olarinmoye AO, Oyinloye JM, Olaoya OB, Edun OA (2011) Distribution, antibiogram and multi-drug resistance in enterobacteriaceae from commercial poultry feeds in Nigeria. Afr J Microbiol Res 5: 294-301.
- Okpokwasili GC, Ogbulie JN (1993) Bacterial and metal quality of tilapia (Oreochromisnilotica) Aquaculture Systems. Int J EnvironHealth Res 3: 190-202.
- Uwaezuoke JC, Ogbulie JN (2008) Microbiological quality of commercially available poultry feeds sold in parts of Eastern Nigeria. Res J Applied Sci 12: 113-117.
- Brown P, Will RG, Brandley R, Asher DM, Dewiller L (2001) Bovine spongiform encephalopathy and variant creutfieltd Jacob disease: Background evaluation and current concerns. Emerg Infect Dis 7: 6-16.
- D hand NK, Joshi DV, Jand SK (1998) Contamination of diary feeds and their toxigenicity. Ind J Animal Sci 68: 1095-1096.
- Ogbulie JN, Okpokwasili GC (1998) Efficacy of Chemotherapeutic agents in controlling bacterial diseases of cultured fish. J Aquaculture Tropics 13: 61-72.
- Arotupin DJ, Kayode R, Awojobi KO (2007) Microbiological and physicochemical qualities of selected commercial poultry feeds in Akure, Nigeria. J Applied Environmental Management 12: 113-117.
- Atere VA, Bamikole AM, Ajurojo OA (2015) Antibiotic Susceptibility of bacteria isolate from poultry feeds sold in Ado Ekiti, Nigeria. J Adv Med life Sci 3: 1-4.
- Healing TO, Greenwood MH (1991) Frequency of isolation of campylobacter spp, yersiniaspp and salmonella spp from small mammals. Int J Environ Health Res 1: 54-62.
- D’Mello JP (2006) Microbiology of animal feeds. Microbiology of Ensilage.
- Aliyu R, Abukakar EO, Adamu MB, Salihu AY, Badai MD, et al. (2012) Bacteriological quality of commercially prepared and self-compounded poultry feeds in Sokoto metropolis. Int J Applied BiolPharma Tech 3: 345-348.
- Alkalaf NA, Osman KA, Ssalama AK (2010) Monitoring of aflatoxins and heavy metals in some poultry feeds. Afr J Food Sci 4: 192-199.
- Krnjaja V, Levic J, Stankovic S (2009) Ubiquity of toxigenic fungi and mycotoxins in animal feeds. BiotechnolAnimHusbandary 25: 455-491.
- David OM, Ogunlade JT (2013) Qualities of feeds produced by local small-scale feed mills in Ekiti State, Nigeria: A public health and feed safety study. Res OpinAnim Vet Sci 3: 297-302.
- Nahid TH (2010) Bacterial contamination in poultry feed in Khartoum State. Department of Microbiology Faculty of Veterinary Medicine, University of Khartoum, Sudan.
- Barnet HL, Hunter BB (1972) Illustrated Genera of Imperfect Fungi (4th Edition), Macmillan Publishing Company, New York, United States 10-12.
- Pearson D (1970) The Chemical Analysis of Food, (7th Edition). Churchill living stones (Edinburg, London).
- AOAC, Association of official analytical Chemists. (1984) Standard methods of analysis of the association of Analytical Chemists. 15th Edition. Washington DC,USA. Pp 5-9.
- Sule IO, Ilori IO (2017) Microbiological Assessment of Poultry Feeds within Ilorin, Nigeria. Notulae Scientia Biologica 9: 34-39.
- Willey J, Sherwood LM, Woolverton CJ (2011) Prescott’s Microbiology (8th Edition) McGraw Hill 1017.
- Obi CN, Ozugbo IJ (2007) Microbiological analysis of poultry feeds sold in Umuahia main market Abia state Nigeria. Res J Applied Sci 2: 22-25.
Copyright: © 2017 Ukaegbu-Obi KM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.