Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2016) Volume 4, Issue 1
The use of herbal medicine as an unconventional health treatment is gaining considerable recognition and popularity worldwide. Despite skepticism and a lack of medical evidence to support its therapeutic efficacy, use of herbal remedies has considerably increased. Belief in the superiority of herbs is based mainly on anecdotal evidence, paraherbalism, and pseudoscience. It is only recently that guidelines for their investigation have been developed and a few herbs have been clinically studied. Many diseases including diabetes mellitus has experimentally been shown to be managed by medicinal plant extracts. The hypoglycemic potential of such plants maybe attributable to the mineral elements present in them. This study was designed to determine the content of mineral elements in five Kenyan antidiabetic medicinal plants traditionally used to manage diabetes mellitus using Total Reflection X-ray Fluorescence (TXRF) System and Atomic Absorption Spectroscopy (AAS) techniques. The elements Mg, K, Ca, Mn, Fe, Zn, Br, Rb, Cr, Ti, Cu, V, Cl and Pb were identified and their contents estimated. The results of the present study provide justification for the usage of these medicinal plants in the management of diabetes mellitus. The results indicates that the analyzed medicinal plants can be considered as potential sources for providing a reasonable amount of the required elements other than diet to diabetic patients. Moreover, these results can be used to set new standards for prescribing the dosage of the herbal drugs prepared from these plant materials.
Keywords: TXRF, AAS, Diabetes mellitus, Trace elements, Mineral elements
Diabetes mellitus describes a group of metabolic disorders characterized by high blood sugar levels and glucose intolerance resulting from defects in insulin secretion, insulin action, or both [1]. The chronic hyperglycemic condition is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels [1]. Several pathophysiological processes are involved in the development of diabetes mellitus [2]. These range from autoimmune destruction of the β-cells of the pancreas [3] with consequent insulin deficiency to abnormalities that result in resistance to insulin action [3]. Deficiency and insufficient action of insulin on target tissues leads to carbohydrates, fats and proteins metabolism abnormalities [4].
The presenting symptoms of hyperglycemia include polyuria, polydipsia, weight loss, sometimes with polyphagia, and blurred vision [5]. In its most severe forms, ketoacidosis or a non-ketotic hyperosmolar state may develop and lead to stupor, coma and, in absence of effective treatment, death [5]. A long standing metabolic derangement is frequently associated with permanent and irreversible functional and structural changes in the cells of the body, with those of the vascular system being particularly susceptible. For instance, retinopathy results in potential loss of vision; nephropathy leads to renal failure; peripheral neuropathy increases risk of foot ulcers, amputations, and Charcot joints; and autonomic neuropathy causes gastrointestinal, genitourinary, and cardiovascular symptoms and sexual dysfunction [6].
The rapidly increasing diabetes mellitus is becoming a serious threat to mankind health in all parts of the world. The mainstay of non-pharmacological treatment of diabetes is diet and physical activity [7]. However, in some cases control and treatment of diabetes and its complications requires conventional therapeutic applications of both insulin and oral hypoglycemic drugs [8]. Conventional management of diabetes is expensive and therefore not affordable by many patients especially in developing nations. More so, conventional drugs are not readily available and have been found to have side effects with long term of use [9]. Control and treatment of diabetes and its complications mainly depend on the chemical or biochemical agents, but the fact is that it has never been reported that someone had recovered totally from diabetes. However, the distinctive traditional medical opinions and natural medicines have shown a bright future in the therapy of diabetes mellitus and its complications.
The hypoglycemic potential of herbal remedies maybe attributable to the mineral elements present in them [10]. These micronutrients are very essential and required by the body in trace amounts or tiny quantities on a day to- day basis in order to function properly. Micronutrients can be categorized into, among others, the macro elements and trace elements. The macro elements include chloride, calcium, phosphorous, magnesium, sodium, potassium, and iron. The trace elements include cobalt, boron, chromium, copper, sulfur, iodine, fluoride, selenium, manganese, zinc, and molybdenum [11].
Macro elements have multiple roles within the body. They work together with vitamins and initiate hormone production as well as speeding up the metabolic processes [12]. Trace elements participate in tissue, cellular and subcellular functions; these include immune regulation by humoral and cellular mechanisms, nerve conductions, muscle contractions, membrane potential regulations, mitochondrial activity, and enzyme reactions [11]. Trace elements interact with vitamins and macro elements to enhance their effects on the body. They are accepted as essential for human health and have diverse metabolic characteristics and functions [13].
Direct associations of macro and trace elements with diabetes mellitus (DM) have been observed in many research studies [14]. Insulin action on reducing blood glucose was reported to be potentiated by some trace elements as chromium, magnesium, vanadium zinc, manganese, molybdenum, and selenium [15]. The proposed mechanism of trace elements enhancing insulin action includes activation of insulin receptor sites, serving as cofactors or components for enzyme systems involved in glucose metabolism [16], increasing insulin sensitivity, and acting as antioxidants preventing tissue peroxidation [17]. It is also reported that the metabolism of several trace and macro elements alters diabetes mellitus and these elements might have specific roles in the pathogenesis and progress of this disease [17].
It is against this background that this study was designed in order to determine the presence, identity and levels of antidiabetic mineral elements in five medicinal plants traditionally used in management of diabetes mellitus in Kenya. The five plants have previously been bioscreened for their antidiabetic potential and found to lower blood glucose levels appreciably in alloxan-induced diabetic mice. It is justifiably postulated that their hypoglycemic potential is due to, among others, antidiabetic mineral elements contained in them.
Study site
This study was undertaken at the Department of Biochemistry and Biotechnology, School of Pure and Applied Sciences, Kenyatta University from July 2013 to February 2015. Kenyatta University is 23 km from Nairobi off Thika Road.
Collection of medicinal plants
The plants used in this study were collected from their native habitats on the basis of ethnobotanical information. They were collected with bioconservation aspects in mind from Kijauri village Nyamira County Kenya. Information on the identity of the plant to collect, the precise locality where it grows, what part to collect, when curative potency is at maximum and the mode of preparation was provided by a traditional medical practitioner. The plants collected and studied were Lippia javanica, Ocimum lamiifolium, Croton macrostachyus, Azadiratchta indica, and Persea americana. For this study, the part of the plants collected were the leaves. Lippia javanica and Ocimum lamiifolium took 21 days to completely dry, Croton macrostachyus took 34 days, Azadiratchta indica took 24 days while Persea americana took 26 days to dry completely. The botanical identity of the plants were authenticated by an acknowledged authority in taxonomy and a voucher specimen deposited at the National Museums of Kenya Herbarium, Nairobi.
Initial preparation of plant materials
Leaves were collected while green and dried at room temperature away from direct sunlight for different periods of time depending on their succulence. The dried leaves were separately ground into fine powder by use of an electric mill. The powdered plant materials were kept at room temperature away from direct sunlight in closed, dry plastic air tight bags ready for extraction.
Extraction
One hundred grams (100 g) of each powdered plant material was later extracted in 1 liter of distilled water at 60°C in a metabolic shaker for 6 hours. The extracts were left to cool, decanted into a clean dry conical flask and then filtered through folded cotton gauze into another clean dry conical flask. The filtrates were stored in a refrigerator at 4°C. The filtrates were then freeze dried using a Modulyo Freeze Dryer (Edward England) in 200ml portions for 48 hours. The freeze-dried powder was then weighed and stored in an airtight container at -20°C.
Elemental analysis
Total reflection x-ray fluorescence (TXRF) system: The TXRF method employs a system where X-rays are made to impinge on the surface of a sample at glancing incidence such that total reflection occurs. The X-rays excite atoms in the top layers of the material and the fluorescence is detected by a detector placed above the sample. The technique is sensitive to very dilute quantities of material. The X-ray source can be an X-ray tube or a synchrotron. This method was used to determine the content of manganese, iron, potassium, calcium, nickel, copper, zinc, strontium, bromine, molybdenum and lead in the aqueous extracts of the five selected medicinal plants.
Sample preparation: One gram (1 g) of each sample was weighed into a clean vial (triplicates) and added 10 mL each of double distilled water. Twenty microliters (20 μL) of 1000 ppm Gallium stock solution was added into each sample (as internal standard) resulting into a concentration of 2 ppm Ga in each sample. Each sample was mixed using a vortex mixer for one minute. Aliquots of 10 μL of each sample were pipetted onto clean quartz carrier using a micro-pipette. Triplicate sub-samples were prepared for each sample. The carriers were then dried in an oven to evaporate the liquid.
Sample spectrum acquisition and quantitative analysis: Each sample carrier was irradiated for 1000 seconds using S2 Picofox TXRF Spectrometer which was operated at 50 kV and a current of 1000 μA. The spectrometer uses a molybdenum anode. Evaluation of the measured spectra was done using S2 Picofox software on the basis of the chosen elements. The concentrations were calculated based on the net intensities of the analyte peak elements and that of the internal standard as per the following formula;
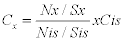
Where, Cx ---- Concentration of the analyte
Cis ---- Concentration of the internal standard
Nx----- Net intensity of the analyte
Nis----- Net intensity of the internal standard
Sx------ Relative sensitivity of analyte
Sis------ Relative sensitivity of internal standard
Quantitative results obtained were copied to an excel worksheet. The worksheet was referred to as raw data. All the data in raw data worksheet was copied to edited worksheet where unnecessary data columns were deleted. Also, unnecessary element row lines were removed. Data in edited worksheet were further evaluated for averages and standard deviations for each set of sub-samples. Average and standard deviation functions in excel were used. Residual data was copied to final worksheet. To these data, all unnecessary columns were deleted leaving the calculated averages and standard deviations values as the final concentrations data.
Atomic absorption spectrophotometry (AAS): This method was used to determine the quantity of magnesium, chromium and vanadium in the plant extracts. Atomic absorption spectrophotometer (AAS) Model: 210VGP (Scientific equipment) was used.
Preparation of reagents
Standard stock solutions: Chromium and magnesium standard stock solutions of 1000 ppm for AAS were used as supplied by the manufacturers (Aldrich Chemical Co., Inc); 1.7852 g of vanadium pento-oxide was dissolved in minimum amount of concentrated sulphuric acid and then heated to dissolve completely, then cooled. The solution obtained was made to 1 liter in a volumetric flask with distilled deionized water. This gave 1000 ppm of the stock solution. Immediately each standard solution was prepared, the flask was thoroughly shaken for mixing and the contents then transferred into a clean plastic bottle and kept in a refrigerator.
Working standards: Suitable aliquots of standard stock solutions of each element were taken in a series of 100 ml volumetric flasks. The solutions were diluted to volume using distilled and deionized water, mixed thoroughly and transferred into plastic beakers. This procedure was done for each element when its analysis was due. Hence, during each analysis fresh working standards were prepared. For each element, working solutions were prepared within a given range where the relationship between the concentration and absorbance was linear. In case of magnesium, 2 ml of 5% lanthanum solution was added to each series of working standards before diluting the standards to volume. In addition, standard blank reagents for Mg, V and Cr were prepared by adding all the used reagents, except the target element being determined.
Lanthanum solution: Lanthanum solution (50 mg/ml) was prepared by dissolving 12.6263 g of lanthanum chloride in distilled deionized water. The solution was then diluted to volume using distilled deionized water in 250 ml volumetric flask. After mixing thoroughly, the solution was kept in clean plastic bottle and used during the determination of magnesium in the plant materials.
Digestion of the plant materials: Each freeze-dried plant material was brought to solution by wet oxidation. In each case, the procedure was repeated twice.
Wet oxidation for determination of Cr, V and Mg: The dried samples weighing 1g were transferred into 100 ml Pyrex beakers and to each beaker; 10ml of concentrated nitric acid was added, and then allowed to soak thoroughly. Three millilitres of perchloric acid (60% HClO4) was added to each beaker, and then warmed on a hot plate slowly, until frothing ceased. Heating was then intensified until all nitric acid was evaporated. When charring occurred, the mixture was cooled, 10 ml of nitric acid was added and heating continued until white fumes of perchloric acid were observed. The final solution was then quantitatively transferred into 100 ml volumetric flask by filtering through Whatman filter paper No. 42. The solutions were then made to volume and shaken well to allow proper mixing before the contents were transferred to plastic sample bottles. For each plant sample, digestion was done in duplicate. The samples were analyzed immediately whenever possible, otherwise kept in refrigerator at -20°C awaiting analyses.
Determination of Cr, V and Mg contents by atomic absorption spectroscopy: The wet digests of the plant materials were analyzed for Cr, V and Mg. The sample solutions for analysis of magnesium were prepared by withdrawing 1ml of the digested sample solution, into 100 ml volumetric flasks. Five milliliters of lanthanum solution was added in each flask and the mixture diluted to volume using distilled deionized water. However, for analysis of Cr and V, the digested sample solutions were analyzed without further dilution. After setting the AAS instrument to the right conditions for each element the respective standards and sample solutions were aspirated into the flame in turns to determine their respective absorbance. At least four standard solutions were aspirated between 6-10 samples to monitor the stability of the working conditions. Distilled deionized water was always flushed into the flame to re-establish the zero absorbance.
For each element, the above procedure was done in duplicate for each sample. The mean absorbance for each sample solution and standard solutions were calculated and recorded. The concentration values obtained were corrected by multiplying with the respective dilution factors. The final values were expressed as μg/g dry matter.
Table 1 shows the mineral element composition of the five aqueous plant extracts traditionally used in the management of diabetes mellitus. Results show that C. macrostachyus contained Mg, K, Ca, Mn, Fe, Zn, Br, Rb, Cr, Ti, Cu, V, Cl and Pb; A. indica contained Mg, Cr, K, Ca, Ti, Mn, Fe, Cu, Zn, Br, Rb, V, Cl and Pb; L. javanica contained Mg, K, Ca, Mn, Fe, Zn, Br, Rb, Ti, V, Cr, Cl and Cu; O. lamiifolium contained Mg, K, Ca, Ti, Mn, Fe, Zn, Br, Rb, Cl, Cu and Pb; and P. americana contained Mg, K, Ca, Mn, Fe, Zn, Br, Rb, Cr, Ti, Cu, V, Cl and Pb.
| MINERAL | PLANTS EXTRACTS | ||||
|---|---|---|---|---|---|
| Croton macrostachyus | Azadiratchta indica | Lippia javanica | Ocimum lamiifolium | Persea americana | |
| Mg* | 206.5 | 213 | 156.5 | 214.75 | 204.5 |
| Fe | 24.90±0.80 | 111±2 | 246±27 | 63.10± 3.00 | 158±2 |
| K | 12737± 255 | 48596± 1815 | 34691±479 | 7131±136 | 55270±1253 |
| Ca | 2148±61 | 1095±44 | 9368±690 | 36788±295 | 21022±167 |
| Mn | 15.3±0.30 | 124±1 | 58.00± 1.30 | 70.30±0.90 | 269±3 |
| Zn | 11.40±0.20 | 62.50±1.80 | 37.10±0.80 | 17.20± 0.42 | 88.30±0.80 |
| Cr* | <0.2 | <0.2 | <0.2 | 0 | <0.2 |
| Cu | 7.64± 0.10 | 0.58±0.04 | 3.54±0.20 | 6.27±0.64 | 3.42±0.15 |
| V* | 3.60± 0.84 | <0.2 | <0.2 | 0 | 17.60±1.20 |
Values of mineral elements with asterisk (*) as a superscript was determined using the AAS. The < means below the limit of detection of TXRF/AAS
Table 1: Mineral elements present in the antidiabetic plants as determined by TXRF and AAS.
Mineral elements are very essential for normal body functioning but are only needed in small quantities [11]. Overdose may upset homeostatic balance or cause toxicity [12] and their deficiency represents an important risk factor for morbidity and mortality in many patients especially those with diabetes mellitus [12]. The hypoglycemic effect of the five studied medicinal plants can justifiably be attributed to, among others, the mineral elements in them especially the trace elements. The study demonstrated the presence of metal ions that have been found in early researches to play vital roles in antidiabetic activity. Analysis of the five plants revealed the presence of Mg, K, Ca, Mn, Fe, Zn, Br, Rb, Cr, Ti, Cu, V, Cl and Pb. Involvement of magnesium in glucose homeostasis is multifactorial. It is involved in multiple levels in insulin’s secretion, its binding and its activity; cofactor in various enzyme pathways involved in glucose oxidation, and it also modulates glucose transport across cell membranes [18]. Magnesium has an important role in the phosphorylation reactions of glucose and its metabolism. Its deficiency has been implicated in insulin resistance, carbohydrate intolerance, dyslipidemia and complications of diabetes [19]. The association between diabetes mellitus and hypomagnesaemia is compelling, because of its wide ranging impact on diabetic control [19].
Manganese is essential for human health. It functions as a key constituent of metallo-enzymes activator in cellular biochemical reactions [20]. It activates an antioxidant enzyme known as manganese superoxide dismutase (MnSOD) that protects the cell membranes and tissues from disruption and degeneration. It helps the body to catabolize lipids, carbohydrates, and proteins and assist in energy production [21]. It also involved in the modulation of glucose transport across cell membranes [22,23]. Manganese deficiency causes impaired glucose tolerance, impaired growth, impaired reproductive function, skeletal abnormalities, and altered carbohydrate and lipid metabolism [10]. Manganese supplements have shown to reverse the impaired glucose utilization induced by manganese deficiency in guinea pigs [24].
Vanadium affects carbohydrate metabolism including glucose transport, glycolysis, glucose oxidation, and glycogen synthesis [25]. At a dose of 100 mg/day vanadyl sulfate improves insulin sensitivity [10]. Its possible mechanism of action in glycemic control is thought to be primarily insulinomimetic with up regulation of insulin receptors. It facilitates glucose uptake and metabolism and enhances insulin sensitivity in animal models and clinically, it has been shown to enhance glucose oxidation and glycogen synthesis, and it modulates hepatic glucose output [23].
Zinc has been shown to be involved in virtually all aspects of insulin metabolism: synthesis, secretion and utilization [26]. It is required as a cofactor for the function of intracellular enzymes that may be involved in protein, lipid, and glucose metabolism [27]. Zinc plays a key role in the regulation of insulin production by pancreatic tissues and glucose utilization by muscles and fat cells [28]. Zinc also influences glyceraldehyde-3-phosphate dehydrogenase, the enzyme involved in glycolysis [29]. Zinc also has a protective effect against β-cell destruction and has anti-viral effects. Diabetics typically excrete excessive amounts of zinc in the urine and therefore require supplementation [22]. Deficiency of intracellular zinc increases beta cell vulnerability to free radical attack. Restoring zinc levels in people with DM would counteract the deleterious effects of oxidative stress. In view of the positive role of zinc on insulin and beta cells, the use of these plants in the treatment of DM may be attributed to considerable amounts of zinc present in them [30].
Calcium is required for normal growth and development of muscles and skeleton [4]. It improves insulin sensitivity in some type 2 diabetic populations [30]. Calcium and cyclic AMP are important in the stimulation of insulin release. The increase in the concentration of ionized cytosolic Ca ions directly mediates the effect of glucose to stimulate insulin release from rat islet of Langerhans [31]. Any alterations in calcium flux can have adverse effects on β-cell secretory function [31].
Potassium supplementation yields improved insulin sensitivity, responsiveness and secretion [32] insulin administration induces a loss of potassium; and a high potassium intake reduces the risk of heart disease, atherosclerosis, and cancer [32,33]. Potassium depletion can result in reduced glucose tolerance [24].
Iron influences glucose metabolism and reciprocally, iron influences insulin action [34]. Impaired glucose metabolism and diabetes mellitus are common clinical manifestations of iron overload in patients with hemochromatosis. Recently, moderately elevated iron stores below the levels commonly associated with hemochromatosis have also been implicated in the etiology of diabetes. Iron interferes with insulin inhibition of glucose production by the liver [35].
Chromium functions as a cofactor in insulin-regulating activities [22]. It facilitates insulin binding and subsequent uptake of glucose into the cell and therefore decreases fasting glucose levels, improves glucose tolerance, lowers insulin levels and decreases total cholesterol in type II diabetic subjects [22,36]. Chromium administration decreases fasting and postprandial glucose and decreases fatigue, excessive thirst, and frequent urination [37]. The regulating or potentiating role of chromium on insulin’s action has been attributed to an increase in insulin binding to cells due to an increase in the number of insulin receptors [12].
Copper is considered as both a powerful enzyme catalyst and a dangerous reactant that generates hydroxyl radical [11]. Copper possesses an insulin-like activity and promotes lipogenesis [11]. Copper is required for absorption and transport of iron and it plays a key role in hemoglobin synthesis [24]. It is associated with hypercholesterolemia and atherosclerosis [24]. A deficiency of copper results in glucose intolerance, decreased insulin response, and increased glucose response [38-40].
The health benefits derived from these mineral elements is a clear indication that their deficiencies appear to be an additional risk factor in the development and progress of disease and they contribute to the pathogenesis of diabetes mellitus and its complications. Their repletion may be an effective therapeutic intervention in prevention of the progression of the diabetes and its complications, along with a glycemic control and control of other risk factors. This study, therefore, established that the studied antidiabetic plants have appreciable quantities of some of the mineral elements associated with glucose lowering effects. It is, therefore, hereby underscored that the antidiabetic potential of these plants can be attributed to, among others, some of the mineral elements present in them.