Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2017) Volume 7, Issue 1
This study was aimed to investigate the influence of age and sex on minerals content and fatty acids profile of Nile Tilapia (Oreochromis niloticus) fillet. Samples were cool transported from Lake Zeway to Food Science and Nutrition laboratory of Addis Ababa University. Minerals content were analyzed using Flame Atomic Absorption spectrophotometer and Fatty acid profile was determined using gas chromatography. Female fish contained significantly (p<0.05) higher calcium and phosphorus as compared to male. Calcium and zinc significantly (p<0.05) decreased as age of fish increased but phosphorus significantly (p<0.05) increased with increase in age of the fish. Sex had no significant effect (p>0.05) on Iron and Zinc content. Monounsaturated fatty acids constituted the largest proportion of total unsaturated fatty acids in both sexes. Oleic acid was relatively higher in female than male. Conversely, the polyunsaturated fatty acids were higher in male as compared to female.
Keywords: Age; Sex; Minerals content; Fatty acids profile; Oreochromis niloticus
Fish is an excellent source of most of the minerals which the body needs to develop properly and perform its functions. Calcium and phosphorus (without which proper development of bones and teeth is impossible) occur in fish fillets in about the same quantities as in beef round [1]. Although fish contain less iron than the amount found in red meat, iron in white fish is well absorbed and so is a useful source of iron. The soft bones of small fish such as sardines and smelts and canned varieties like salmon are especially valuable sources of calcium. Fish is a rich source of protein, fatty acids, and essential vitamins and minerals such as vitamin A, calcium, iron, zinc, and iodine. The vitamin A, calcium and iron found in small fish species are particularly bio available that is, easily absorbed by the body [2]. Fish absorb minerals not only from their diets but also from the surrounding water [3]. Exogenous factors that affect fish body composition include the diet of the fish (composition, frequency) and the environment in which it is found (salinity, temperature). The main exogenous factor affecting proximate composition is diet. Various studies have examined the effects of temperature, light, salinity, pH and oxygen concentration on the proximate composition of fish but these factors would seem to have very limited effects. On the other hand, endogenous factors are genetic and linked to the life stage, age, size, sex and anatomical position in the fish [3]. These endogenous factors govern the majority of principles that determines the composition of fish [3]. A remarkably variation in the lipid and fatty acid contents of the herbivorous fish, Oreochromis niloticus, was found in the five Ethiopian study lakes [4]. This study supports findings from temperate lake studies which show that the variability of fatty acid and lipids can be great both within and between species. Temperate lake studies show that the variability of fatty acid and lipids can be great both within and between species [4]. Possible factors affecting the abundance and composition quality of lipids and fatty acids in fish is genetic variation, size of fish, season, water and temperature [3]. Therefore, in view of this fact, the present research was carried out to determine minerals contents and fatty acids of Nile Tilapia fillet.
Description of the study area
Lake Zeway is the most northerly rift valley lakes of Ethiopia. It is endowed with different kind of fish species like Nile Tilapia (Oreochromis niloticus) (Linnaeus 1758), African Catfish (Clarias gariepinus) (Burchell 1822), Crucian carp (Carassius carassius) (Linnaeus 1758), Ethiopia Barb (Labeobarbus intermidus) (Rüppell 1835), Barbus paludinosis, Zilli´s cichlid Tilapia zillii (Gervais 1848) and Gara species.
Sample collection and preparation
Fresh fish was purchased from local fishermen at Bochessa, Korokonch and Menefesha landing sites of Lake Zeway. Live fish was transported to Batu Fishery and Aquatic Life Research Center laboratory layered with flaked ice using ice box. Sex was identified by examining genital papilla located immediately behind the anus. In male the genital papilla has only one opening (the urinary pore of the ureter) through which both milt and urine pass [5]. In female the eggs exit through a separate oviduct and only urine passes through the urinary pore. Immediately after sex identification, length of fish was measured to the nearest 0.1 cm and converted to age using Von Bertalanffy Growth Function [6].
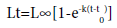
In the above Von Bertalanffy Growth Function equation constant parameters like L, K and to for female are 30.19 cm, 0.25 per year and –0.27 year respectively and for male are 30.81 cm, 0.27 per year and –0.12 year respectively have been established [7]. To determine age, 19.08 cm and 22.10 cm for female 20.6 cm and 23 cm length for male were selected and inserted into the above Von Bertalanffy Growth Function. After sexes have identified and age was determined, composite of fish sample from the same age and sex was cleaned, descaled, eviscerated and filleted manually using sterile plastic knife. The fillet was oven dried at 60ºC for 72 h then transferred into desiccators and cooled for 30 min. Dried fillet was grounded to 0.3 mm size using laboratory mill and the powder was stored in desiccators for further proximate composition analysis.
Minerals analysis
The amount of minerals was analyzed using the following formula [8].
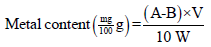
Where: W: Weight of the sample (g), V: Volume of the extract (mL), A: Concentration (μg/mL) of sample solution, B: Concentration (μg/mL) of blank solution. Phosphorous was determined by the colorimetric method using ammonium molybdate. Phosphorous was calculated using the following formula:
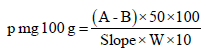
Where: A: Reading of the sample solution, B: Reading of the blank solution, W: Weight of fresh sample.
Determination of fatty acids profile
Lipid was extracted using the procedure [9]. Fatty acid methyl ester (FAME) was prepared according to alkaline based transesterification. The fatty acid methyl esters were analyzed by Dani Italia (GC-1000) gas chromatography with a Flame Ionization Detector (FID) and capillary column (30 m length × 0.32 mm internal diameter). The oven temperature was 50ºC, held 2 min, and raised to 250ºC at a rate of 4ºC/min and detector temperature was 260ºC. The carrier gas used was nitrogen. The injected sample volume was 0.3 μ. The fatty acids methyl esters were identified by comparing the retention time of the samples and appropriate fatty acids methyl esters standards [10]. Retention time and peak areas were recorded with the help of clarity chromatography software.
Phosphorus and calcium contents
From Table 1, age, sex and the interaction effect of age and sex significantly (p<0.05) affected phosphorus and calcium contents. Phosphorus significantly (p<0.05) increased from 16.4 mg/100 g to 17.1 mg/100 g and calcium decreased significantly (p<0.05) from 38.2-27.3 mg/100 g with the increase of fish age from four to five years. Female fish contains higher phosphorus (17.2 mg/100 g) and calcium (35.0 mg/100 g) than male phosphorus (16.5 mg/100 g) and calcium (30.5 mg/100 g). Arannilewa et al. [11], reported the average phosphorus content of Nile Tilapia as 15.32 mg/100 g which is very similar with the present study and higher calcium contents (61.67 mg/100 g). Amer [12] reported that there is higher phosphorus (0.09%) in female than male (0.086%).
| Parameter | Phosphorus (P) | Iron (Fe) | Zinc (Zn) | Calcium (Ca) | |
|---|---|---|---|---|---|
| Sex | Male | 16.5 ± 0.06b | 1.61 ± 0.02a | 0.5 ± 0.01a | 30.5 ± 0.10b |
| Female | 17.2 ± 0.07a | 1.62 ± 0.02a | 0.5 ± 0.01a | 35.0 ± 0.10a | |
| Age | Four | 16.4 ± 0.07y | 1.63 ± 0.02x | 0.6 ± 0.01x | 38.2 ± 0.10x |
| Five | 17.1 ± 0.07x | 1.60 ± 0.02x | 0.5 ± 0.01y | 27.3 ± 0.10y | |
Table 1: Mean ± SE minerals contents of Nile Tilapia (O. niloticus) fillet in mg/100 g in fresh weight basis.
Iron content
The Iron content of fish muscles was not affected (p>0.05) by both age and sex but the interaction effects of age and sex significantly (p<0.05) affected iron content. Iron content was slightly higher in female (1.62 mg/100 g) than in male (1.61 mg/100 g). Iron content was decreased with increase of age. Petenuci et al. [13] reported 1.3 mg/100 g of iron in Nile Tilapia. The major factors influencing iron absorption in fish are the proportion of organic and inorganic components of the diet, the amount of feed ingested and the conditions of the digestive tract of fish [1].
Zinc content
Age significantly (p<0.05) affected zinc contents where as sex and the interaction effect of age and sex has no significant (p>0.05) effect on zinc content. Zinc content has decreased from 0.6-0.5 mg/100 g with the increase of fish age. The absorption and amounts of zinc in fish may be affected by the chemical form of Zinc in the diet, the source of protein and the presence of other dietary components such as Calcium, Phosphorus and Phytic acid [1].
Fatty acids profiles
From Table 2, seven different fatty acids were identified. There were several unidentifiable peaks which were sum up and marked as Σ unidentified fatty acids for which standard is not available. Total fatty acid was calculated from the total integrated areas of all fatty acids in the chromatograms. Irrespective of age and sex, fatty acids identified from Nile Tilapia fillet were Myristic, Palmitic, Stearic, Oleic, Linoleic, Linolenic and Heneicosanoic. The fatty acids composition of fish tissue can be affected by diet, size, age, reproductive cycle, salinity, temperature, season and geographical location p [1,14].
| 14:0 | 16:0 | 18:0 | 21:0 | SSAFA | 18:1ω9 | SMUFA | 18:2ω6 | 18:3ω3 | SPUFA | Sunid | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | |||||||||||
| Male | 4 | 0.6 | 26.1 | 3.7 | 14.9 | 45 | 13.9 | 13.9 | 5.2 | 11 | 16.2 | 24.9 |
| 5 | 2.5 | 24.2 | 5.8 | 7.1 | 39.6 | 23.9 | 23.9 | 5.6 | 8.9 | 14.5 | 22 | |
| Female | 4 | 2.5 | 25.5 | 4.1 | 10.5 | 42.6 | 34.9 | 34.9 | 6.5 | 8.3 | 14.8 | 7.7 |
| 5 | 3.7 | 27.1 | 5.5 | 5.4 | 41.7 | 24.1 | 24.1 | 5.4 | 9.3 | 14.4 | 19.5 | |
Table 2: Percentage fatty acid composition of Nile Tilapia (O. niloticus) fillet from Lake Zeway.
Saturated fatty acids
From Table 2, the percentage of saturated fatty acids was 45% and 39.6% in four and five year male fish and 42.6% and 41.7% in four and five year female fish. The most abundant saturated fatty acids in animal and plant tissues are straight-chain compounds with 14, 16 and 18 carbon atoms. Myristic acid (14:0) is a ubiquitous component of lipids in most living organisms. Palmitic acid (16:0) is usually considered the most abundant saturated fatty acid in nature, and it is found in appreciable amounts in the lipids of animals, plants and lower organisms. It comprises 20-30% of the lipids in most animal tissues, and it is present in amounts that vary from 10-40% in seed oils [15]. As reported by Ackman [10], palmitic acid as key metabolite not influenced by diet in fish, was the saturated fatty acids found in higher concentration [16]. Stearic acid (18:0) is the second most abundant saturated fatty acid in nature, and again it is found in the lipid of most living organisms [15]. Akpinar et al. [17], reported that the major saturated fatty acids in male and female salmon trutta was palmitic acids. Study conducted by Suloma et al. [18], shown that the predominant saturated fatty acids in Nile Tilapia was palmitic.
Monounsaturated fatty acid
The percentage of monounsaturated fatty acid was 13.9% and 23.9% in four and five year male fish and 34.9% and 24.1% in four and five year female fish. Oleic acid is the main monounsaturated fatty acids common to both sexes with higher concentration in female than male. Satue and Lopez [16] reported oleic acid was higher in female rainbow trout as compared to male. Monounsaturated fatty acids constituted the largest proportion of total unsaturated fatty acids in both sexes.
Polyunsaturated fatty acids
The percentage of polyunsaturated fatty acids were 16.2% and 14.5% in four and five year male and 14.8% and 14.4% in four and five years female fish. Relatively similar average results were reported by various renowned researchers [13,14,18-21]. The Polyunsaturated fatty acids was higher in male rainbow trout as compared to female for the similar age but the vice versa is true for monounsaturated fatty acids Satue and Lopez [16] was reported. The lower polyunsaturated fatty acids in female may related to egg formation [16]. Fatty acids contents of lipid in fish are generally reported to vary considerably, both within and between species [22-24]. A pronounced difference in the fatty acid composition of O. niloticus fillet is due to differences in maturation processes that exist between the two sexes [25].
<p>From the present study it can be concluded that, there is variation between age and sex of Nile Tilapia with regard to minerals contents and fatty acid profile. Eating fish with high contents of polyunsaturated fatty acids is believed to be important for human health in reducing the risk of cardiovascular disease and diabetes. In this case male Nile Tilapia is good source of polyunsaturated fatty acid. Female fish contains higher monounsaturated fatty acids than males.</p>
I would like to acknowledge rural capacity building project for financial support of the project through Ethiopian Institute of Agricultural Research (EIAR).