Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Research Article - (2022)Volume 11, Issue 4
Background: Various drugs have been studied in the management of COVID-19 infection and the only antiviral that has been proven efficacious is Remdesivir which is a broad spectrum antiviral agent having activity against RNA dependent viral RNA polymerase.
Objective: To compare the frequency of mortality in patients of COVID-19 when given Remdesivir in early stages of the disease as compared to the late stages of the disease.
Study design: Retrospective comparative cross sectional study. Study setting Farooq hospital Lahore, Pakistan.
Study duration: 12 Months.
Data analysis: All patients admitted from January 2020 till December 2020 above 18 years of age of either gender diagnosed COVID-19 positive by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) with moderate illness, having classical radiological lesions of COVID-19 on X-ray chest or High-Resolution Computed Tomography (HRCT) chest, respiratory rate>22/min and >50% of radiological involvement of lung with typical lesions, deranged ≥ 2 biochemical markers CRP>20 mg/l, LDH>600 U/L, D Dimers>500 ng/ml, serum ferritin<500 ng/ml were enrolled. Patients who died before completion of therapy, required invasive mechanical ventilation at the time of presentation, and those patients who also received other experimental therapies, including convalescent plasma, tocilizumab or thrombolytic were excluded from the study. Data was entered and analyzed using SPSS 22. Quantitative variables were presented as mean and standard deviation while qualitative variables were presented as frequency and percentages. The difference between means of continuous variables was assessed using t-test/Mann Whitney-U test and difference between proportions of categorical variables, using Fisher’s exact test/Chi-square test. Differences in outcomes between the study groups were considered significant if p<0.05 (two-sided).
Results: In our study a total of 111 patients were enrolled with mean age of 44.25 ± 14.3 years (18-78 years). In these patients Remdesivir was started in less than 5 days, within 5-10 days and greater than 10 days with mortality of 27.7%, 40.5% and 63% respectively.
Conclusion: Our study concludes that remdesivir treatment is more effective when started in early course of treatment as compared to when its initiation was delayed. All patients presenting with moderate to severe disease should be started with remdesivir soon after the onset of symptoms which would result in decreased mortality.
COVID-19 infection; Antiviral agent; Remdesivir; Mortality
On 12th December 2019, a patient was hospitalized with severe pneumonia of unknown etiology, in Wuhan, Hubei province, China. Clusters of similar cases were spreading within the province, and, in early January 2020, the unidentified pneumonia was discovered to be caused by a novel viral subtype of the Coronaviridae family, designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1-3]. By the end of January 2020, WHO declared SARS-CoV-2 infection as a public health emergency of international concern and a few days after, officially named the disease as “coronavirus disease 2019” (COVID-19). The pandemic state was declared on the 12th of March 2020, and, since then, the outbreak exponentially advanced worldwide. Although mild respiratory tract infection characterizes most COVID-19 cases with cold symptoms, it is a serious global situation [4]. Severe outcomes usually occur in older male patients with secondary comorbidities [5].
The incubation period for COVID-19 is estimated to be four days (interquartile range: 2-7 days). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a betacoronavirus which is a 29,903 bp single-stranded RNA virus that targets the Angiotensin-Converting Enzyme (ACE)-2 receptors to gain entry into the host cells. The ACE-2 receptor is widely present in capillaries of respiratory and nervous systems [6]. Frequently- reported symptoms of patients admitted to the hospital with COVID-19 infection include fever, cough, myalgia, fatigue, and shortness of breath (3%-31%) at illness onset. Approximately 20%-30% of patients hospitalized with COVID-19 and pneumonia have required respiratory support in Intensive Care Units (ICUs) [7].
In the light of an uncontrolled expansion and the steadily increasing COVID-19 fatalities in January and February 2020, huge efforts were put into the identification of effective antiviral agents against COVID-19. Nucleoside/nucleotide analogs are one of the most promising antiviral drug classes in general, and significant drug discoveries emerged from this class that today form the basis of treatment against the infection [8].
Remdesivir or GS-5734 is a prodrug of a nucleoside analog with direct antiviral activity against several single-stranded RNA viruses, including SARS-CoV and Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Remdesivir is a monophosphoramidate prodrug of an adenosine analogue and it has a broad-spectrum antiviral activity against paramyxoviruses, falviviruses and coronaviruses. It showed in vitro activity on human airway epithelial cells against SARS-CoV-2. It is an investigational drug and was granted an emergency use authorization by Food and Drug Administration (FDA), so it is still under clinical trial. The potent mechanism of action of this drug is still unclear but it exercises its effects through several processes.
It can interfere with nsp12 polymerase even when exoribonuclease proofreading is intact. It can also produce Nucleoside Triphosphate NTP that acts pharmacologically active alternate substrate of RNA-chain terminator, as a result NTP can constrain active triphosphates into viral RNA of coronaviruses. There is evidence of high genetic barrier to develop resistance against Remdesivir in coronavirus as a result of which it maintains its effectiveness in antiviral therapies against these viruses. Effectiveness of Remdesivir has been reported against different groups of coronaviruses including Alphacoronavirus NL63 and several SARS/MERS-CoV coronaviruses [9].
The initial EUA of remdesivir was based on preliminary data analysis of the Adaptive COVID-19 Treatment Trial (ACTT) which was announced on April 29, 2020. The final analysis included 1,062 hospitalized patients with advanced COVID-19 and lung involvement, showing that patients treated with 10-days of remdesivir recovered faster than similar patients who received placebo. Results showed that patients who received remdesivir had a 31% faster time to recovery compared with those who received placebo (P<0.001). Specifically, the median time to recovery was 10 days in patients treated with remdesivir compared with 15 days in those who received placebo (P<0.001). Patients with severe disease (n=957) had a median time to recovery of 11 days compared with 18 days for placebo. A statistically significant difference was not reached for mortality by day 15 (remdesivir 6.7% vs. placebo 11.9%) or by day 29 (remdesivir 11.4% vs. placebo 15.2%) [10].
The rationale of this study is to determine the effectiveness of remdesivir during the disease course because its beneficial role has been established with good outcomes but still there is lack of data regarding initiation of remdesivir therapy. There is no clear cut off point which indicates when to start remdesivir weather at an initial stage or later or in the terminal stage. Inconsistent data about the efficacy of remdesivir is attributable to various factors including the heterogeneous nature of COVID-19 treatment in the pandemic as the treatment options evolved, lack of clarity of the optimal timing of drug initiation, treatment efficacy assessed from either ‘symptom onset’ or ‘diagnosis onset,’ and difference in ‘standard of care’ across the globe. Specifically, the relationship between timing of remdesivir initiation (from symptom onset) and outcomes has not been clearly elucidated. There is a need to evaluate the effect of optimal remdesivir initiation timing that would improve outcomes in COVID-19, especially in the subsets of moderate-to- severe disease. This study aimed to evaluate the impact of shorter Symptom Onset to Remdesivir Treatment (SORT) interval on clinical outcomes in the subsets of moderate-to- severe COVID-19. We did this retrospective trial to compare mortality when remdesivir was started at an early stage and when given in advanced disease.
This retrospective comparative cross sectional study was conducted at a dedicated COVID ward in Farooq Hospital, Lahore. Ethical approval was taken from the hospital ethical review Committee, consent was not taken from the patients as de-identified patient data were used for analysis. All patients admitted from January 2020 till December 2020 above 18 years of age of either gender diagnosed COVID-19 positive by RT-PCR with moderate illness, having classical radiological lesions of COVID-19 on X-ray chest or HRCT chest, respiratory rate>22/min and>50% of radiological involvement of lung with typical lesions, deranged ≥ 2 biochemical markers CRP>20 mg/l, LDH>600 U/L, D Dimers>500 ng/ml, serum ferritin<500 ng/ml were enrolled. Patients who died before completion of therapy, required invasive mechanical ventilation at the time of presentation, and those patients who also received other experimental therapies, including convalescent plasma, tocilizumab or thrombolytics were excluded from the study.
Data regarding age, gender, co-morbidities like diabetes, hypertension, chronic kidney disease and chronic liver disease was entered. Baseline CRP, ferritin level, LDH levels and severity of the disease were noted. All admitted patients were given a loading dose of 200 mg followed by 100 mg once a day for 5-10 days. The timings of injection remdesivir was noted. Patients received intravenous remdesivir 200 mg on day 1 as loading dose, followed by a maintenance dose of 100 mg daily for a total of 5-10 days. Patients also received other drugs as per protocol, including corticosteroids, anticoagulants, and other supportive therapy. Mortality during index hospitalization was noted. Data on clinical and laboratory findings, including mortality, Length of Hospital Stay (LOHS), and safety outcomes Adverse Events (AEs) and suspected drug-related hypersensitivity reactions were extracted from the medical records. Data was entered and analyzed using SPSS 22. Quantitative variables were presented as mean and standard deviation while qualitative variables were presented as frequency and percentages. Patients were divided in three arms, one who received remdesivir within 5 days of onset of symptoms, second arm received remdesivir within 5-10 days of symptoms and third who received after 10 days. The difference between means of continuous variables was assessed using t-test/Mann Whitney-U test and difference between proportions of categorical variables, using Fisher’s exact test/Chi-square test. Differences in outcomes between the study groups were considered significant if p<0.05 (two-sided).
In our study total 111 patients were enrolled with mean age of 44.25 ± 14.3 years (18-78 years). There were 55.9% (62) males and 44.1% (49) females. Moderate disease was present in 29.7%(33) patients and severe disease was present in 70.3% (78) patients (Figures 1 and 2). Most common co-morbidity was hypertension in 62.2% patients, ischemic heart disease in 41.4% patients, COPD in 30.6% patients, diabetes in 29.7% patients and CKD in 25.2% patients (Tables 1 and 2 ).
| Effect of remdesivir | In-hospital mortality | Total | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| Interval of starting remdesivir | Less than 5 days | Count | 13 | 34 | 47 |
| % | 27.7% | 72.3% | 1% | ||
| 5-10 days | Count | 15 | 22 | 37 | |
| % | 40.5% | 59.5% | 1% | ||
| More than 10 days | Count | 17 | 10 | 27 | |
| % | 63% | 37% | 1% | ||
Note: p-value 0.01 significant`
Table 1: Effect of initiation of remdesivir in hospital mortality.
| Interval of starting remdesivir | N | Minimum | Maximum | Mean | Std. deviation |
|---|---|---|---|---|---|
| Less than 5 days | 47 | 5 | 18 | 9.19 | 3.392 |
| 5-10 Days | 37 | 5 | 19 | 10.65 | 2.87 |
| More than 10 days | 27 | 5 | 21 | 13.3 | 4.77 |
| ANOVA <0.001 significant | |||||
Table 2: Effect of remdesivir initiation time on length of hospital stay.
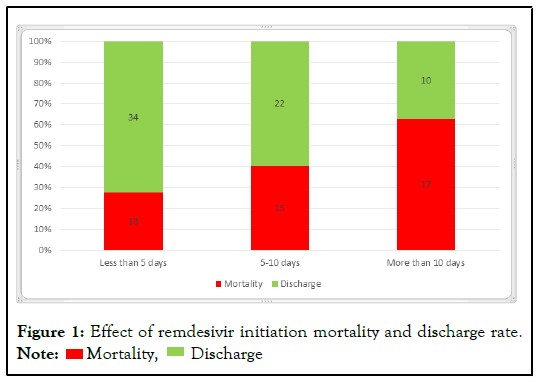
Figure 1: Effect of remdesivir initiation mortality and discharge rate. Note:  Mortality,
Mortality,  Discharge
Discharge
Figure 2: Effect of remdesivir initiation time on length of hospital stay.
This study was conducted at Farooq hospital Lahore. Farooq hospital has the biggest COVID isolation center in the private sector in the city of Lahore. While the pandemic reached its peak, the medical fraternity was struggling with different trials of treatment based on their previous experiences of treating other viral infections. Different hospitals were following different treatment strategies based on 'whatever they liked or presumed to be suitable'. In this region where not too many anti virals were easily available and the cost was also a limiting factor, we chose Remdesivir against other drugs like tocilizumab to study its benefits at different stages of the disease. Tocilizumab was another drug in vogue at that time but was very expensive as compared to Remdesivir and wasn't easily available. COVID-19 effects several countries worldwide and its treatment options are not well defined and a lot of studies focus only on the efficacy of remdesivir by comparing its treatment timings with a placebo affect [11,12].
In our study it was found that remdesivir was more effective when started in early course of the treatment as compared to starting it late (p-value<0.05). Similar results were seen in other studies. In the randomized, double-blinded placebo-controlled Adaptive COVID-19 Treatment Trial (ACTT-1), the trial shows a strong mortality rate in patients of prespecified group treated with RDV while requiring low flow oxygen therapy [13]. The golden time period in which maximum efficacy of antiviral drugs can be achieved is the inital stage of viral replication. Moreover as interval between inoculation of virus and onset of symptoms is five days, beginning the treatment early is of utmost importance. Our study indicates that the shorter the distance between symptoms onset and remdesivir treatment the lesser the mortality rate. 346 patients were included in the final study out of 350 patients who received remdesivir. Most of these patients were male with average age of 60. Kapler-meier study shows that there is a large difference in the survival of patients with SORT therapy of less than nine days and those of more than nine days. A significant lower mortality rate was observed in patients with less than nine days SORT compared to the later. Similarly, the length of hospital stays, and odds of death were much less in shorter SORT intervals.(P=0.34 and P=0.03 respectively) [14].
Study of Goldmen, et al. shows that the hospital discharge rate was high in patients who received first dose of remdesivir within less than ten days of symptoms onset than those who received first dose after 10 days of symptoms onset in patients not requiring mechanical assistance [15].
Robert, et al. [16] enrolled a total of 562 patients who underwent randomization and received at least one dose of remdesivir or placebo were included in the analysis-279 patients in the remdesivir group and 283 in the placebo group. The mean age was 50 years, 47.9% of the patients were women, and 41.8% were Hispanic or Latinx. The most common coexisting conditions were diabetes mellitus (61.6%), obesity (55.2%), and hypertension (47.7%). COVID-19 related hospitalization or death from any cause occurred in 2 patients (0.7%) in the remdesivir group and in 15 (5.3%) in the placebo group (hazard ratio, 0.13; 95% Confidence Interval (CI), 0.03 to 0.59; P=0.008). A total of 4 of 246 patients (1.6%) in the remdesivir group and 21 of 252 (8.3%) in the placebo group had a COVID-19 related medically attended visit by day 28 (hazard ratio, 0.19; 95% CI, 0.07 to 0.56). No patients had died by day 28. Adverse events occurred in 42.3% of the patients in the remdesivir group and in 46.3% of those in the placebo group. Among non-hospitalized patients who were at high risk for COVID-19 progression, a 3-day course of remdesivir had an acceptable safety profile and resulted in an 87% lower risk of hospitalization or death than placebo. The first stage of the Adaptive COVID-19 Treatment Trial (ACTT-1) showed that patients with moderate-to-severe COVID-19 who were treated with remdesivir had a shorter time to recovery and a lower risk of progression to more severe respiratory disease than patients who received placebo [17]. One of the simple trials (GS- US-540-5774) showed that patients with moderate COVID-19 who received remdesivir for up to 5 days had significantly higher odds of having a better clinical status on day 11 [18].
The analysis of change in the mortality rate by variable initiation of treatment with remdesivir is the major difference between our study and other trials. Older studies suggest about average gap of eleven days between symptoms onset and onset of treatment for different outcomes. Our study proposes the need to initiate treatment closer to the onset of symptoms.
Our study concludes that remdesivir treatment is more effective when started in the early course of treatment as compared to delayed initiation. All patients presenting with moderate to severe disease should be started with remdesivir soon after the onset of symptoms which would result in decreased mortality.
Citation: Manzoor Z, Khan HI, Abidin SU, Maqsood F, Khan AF, Sikandar SM, et al. (2022) Mortality in Patients of COVID-19 when given Remdesivir in Early Stages of the Disease as Compared to Late Stages of the Disease in Pakistani Population. J Develop Drugs. 11:175.
Received: 06-Jul-2022, Manuscript No. EOED-22-18069; Editor assigned: 11-Jul-2022, Pre QC No. EOED-22-18069 (PQ); Reviewed: 21-Jul-2022, QC No. EOED-22-18069; Revised: 28-Jul-2022, Manuscript No. EOED-22-18069 (R); Published: 08-Aug-2022 , DOI: 10.35248/ 2329-6631.22.11.175
Copyright: © 2022 Manzoor Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.