Research - (2022)Volume 7, Issue 4
Purpose: High mortality and high heterogeneity are main characteristics of colorectal cancer, whose prognostic predictive indexes are not clear enough. This study aims to elucidate the value of mucinous content as a prognostic parameter for stage I-III colorectal cancer patients.
Methods: This was a retrospective study of 3,852 patients with stage I-III colorectal adenocarcinoma, adenocarcinoma with mucinous content, and mucinous adenocarcinoma (grouped by their mucinous content, 1% and 50% was the cutoff) who underwent curative surgery. Survival curves were plotted by the Kaplan-Meier method, and the differences were evaluated by the log-rank test. Multivariate analyses of oncological outcomes were performed by the Cox proportional hazard model to determine whether mucinous content can independently predict prognoses after corrections. The Akaike information criterion values were obtained to compare the predictive value. Baseline variables were also examined.
Results: After correcting for confounding factors, high mucinous content was found to be an independent predictor for negative overall survival (adjusted HR AMC=1.351, adjusted HR MAC=4.142) and negative disease-free survival (adjusted HR MAC=1.968). Mucinous adenocarcinomas implicated the worst prognoses. Mucinous content had the second-highest predictive value for patient death (AIC=13779.547) and the fifth-highest predictive value for tumor recurrence/distant metastasis (AIC=14052.415) among the analyzed variables. Furthermore, each histopathological subtype had unique clinicopathological features.
Conclusion: Mucinous content can group stage I-III colorectal cancers with regard to clinicopathological characteristics and oncological outcomes, whose prognostic value was greater than many other parameters. Mucinous content is a vital clinical reference.
Mucinous content; Prognostic parameter; Colorectal cancer; Clinicopathological characteristics; Oncological outcomes
OA: Osteoarthritis; CDC: Centers for Disease Control; ROM: Range Of Motion; SLSD: Single Leg Step Down; KOOS: Knee Injury Osteoarthritis and Outcome Score; ArJD: Activity-related Joint Pain; GCP: Good Clinical Practice; QOL: Knee-related Quality Of Life; ADL: Activities of Daily Living; ANCOVA: Analysis of Covariance; ITT: Intention-To-Treat population; LOCF: Last Observation Carried Forward; Treg: T regulatory; DRKS: Deutsches Register Klinischer Studien; ICH: International Council for Harmonisation
Colorectal Cancer (CRC) is a deadly and commonly diagnosed cancer worldwide [1,2]. High heterogeneous is also one of its representative characteristics, which limits the treatment. However, the Tumor Node Metastasis (TNM) stage is still the primary reference when selecting management strategies for CRC patients, especially when determining adjuvant chemo-radiotherapy methods [3-5], although survival paradoxes have been widely found. Therefore, exploring and validating a new clinicopathological indicator is becoming increasingly vital. Mucinous content is a potential histological indicator, whose first mention can be traced back to 1923. It is the lesion either malignant gland closely associated with and thereby likely producing Mucin (MUC) or sizable mucin pools forming part of the tumor volume, which could be found in CRC [6-9] and other cancers. Its clinical effects have been widely shown [10-14]. For CRCs, based on the mucinous content, tumors could be divided into adenocarcinoma (AC, tumor with less than 1% mucinous differentiation), adenocarcinoma with mucinous composition (AMC, adenocarcinoma with intermediated mucinous component), and Mucinous Adenocarcinoma (MAC, carcinoma with greater than 50% mucinous content) [9]. Related studies are underway. Compared with AC, MAC has been found to be a distinct histological subtype of CRC, accounting for 10%–15% [15,16]. It has poor prognoses [17,18], unique gene mutation sites, and poor responses to cytotoxic chemotherapy and radiotherapy [19,20]. AMC was also shown to be a unique CRC subtype, although its correlated studies were fewer than those performed on MAC. The characteristics of AMC in oncological outcomes [21], genomic landscape, and clinical features [22-24] have been found. However, there are still controversies regarding the prognostic value of mucinous content [25-30]. The distinctions among the three histological subtypes were also unclear, and AMC patients would even be simply considered AC patients [31]. These ambiguities limit the clinical applications of mucinous content. Needed to be systematically elucidated.
To determine the prognostic and classified value of mucinous content, we reevaluated the mucinous content in histological slices, grouped CRCs accordingly, and compared the oncological outcomes and clinicopathological characteristics of different pathological subgroups in patients with stage I-III CRC who underwent curative resection in a large sample size. We showed the high prognostic value of mucinous content and compared its predictive value with other variables to further highlight its clinical reliability. In most cases, possible prognoses are great clinical references; moreover, tumors clinicopathological characteristics might also provide windows for patient management. Our work systematically and comprehensively explored the value of mucinous content and controlled the methodological drawbacks of previous studies as much as possible. This work also mentioned the Multidisciplinary Teams (MDTs).
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes and was reviewed and approved by the Ethics Committee of the Affiliate Hospital of Qingdao University (reference number: QYFY WZLL26486). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. The need for written informed consent was waived by the Ethics Committee of the Affiliate Hospital of Qingdao University due to retrospective nature of the study.
Patient selection
We retrospectively included patients who underwent curative resections for primary colorectal AC, AMC, and MAC in stages I to III at the Affiliated Hospital of Qingdao University from 2001 to 2020. Patients were identified by their unique medical record number through the hospital information system. Patients with preoperative anticancer treatments (n=874), personal cancer history (n=41), positive margins (n=112), and missing data (n=413) were excluded. Ultimately, there were a total of 3,852 patients in this study.
Feature selection
Selected features were as follows: histological type (AC vs. AMC vs. MAC), age (60 was the cutoff), sex, Body Mass Index (BMI, 28 was the cutoff), Hypertension (HP), Chronic Heart Disease (CHD), Diabetes Mellitus (DM), smoking history, drinking history, family history of tumors, family history of gastroenterology tumors, serum Carcinoembryonic Antigen (CEA) level, Serum C-reactive Protein (CRP) level, tumor position (right colon vs. left colon vs. rectum), tumor size (diameters, 20 mm was the cutoff), lesion amount (multifocal vs. unifocal), surgical therapy (laparotomy vs. laparoscopy), tumor differentiation grade (differentiated vs. undifferentiated), Ki-67 protein level, Perineural Invasion (PNI), Lymphovascular Invasion (LVI), and TNM stage [32].
Outcome selection
Composite outcomes were used. The implication of each feature for Overall Survival (OS) and Disease Free Survival (DFS) was the primary outcome. OS was defined as the date of surgery to the date of death or the follow-up deadline (April 30, 2021). DFS was defined as the date of surgery to the date of tumor recurrence/ distant metastasis or the follow-up deadline (April 30, 2021). The secondary outcome was the fit of variables to patient prognoses.
Histological re-evaluation
The appearance of AC, AMC, and MAC under the microscope are shown in Figure 1. The mucinous content of each slice was carefully reevaluated under hematoxylin-eosin (H&E) staining. Tumors were grouped based on the average mucinous content (at least 3 slices/tumor, average 5 slices/tumor). All tumor tissues were independently assessed by at least two experienced pathologists who were blinded to previous pathological reports and clinical parameters. When there were any objections, a third pathologist (or more) joined the assessment process. Majority decisions were considered the final. Moreover, the percentage of observed mucinous composition was determined by gross specimen rather than endoscopic biopsy to prevent deviations caused by insufficient samples. The Ki-67 protein level, which showed the invasiveness of tumors, was also reevaluated [33].
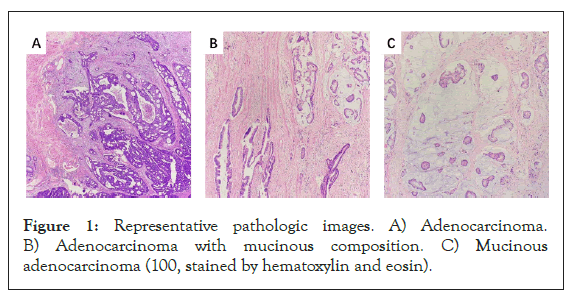
Figure 1: Representative pathologic images. A) Adenocarcinoma. B) Adenocarcinoma with mucinous composition. C) Mucinous adenocarcinoma (100, stained by hematoxylin and eosin).
Postoperative follow-up
Postoperative outcomes were investigated through routine scheduled outpatient services at 3-month intervals during the first 2 years, at 6-month intervals during the 2-5 years and at 12-month intervals, thereafter, including examinations as follows: Medical history collection, physical examination, serum tumor marker levels, abdominal and pelvic computed tomography, and colonoscopy. In addition, telephone interviews were also used.
Statistical analysis
Survival curves were plotted by the Kaplan-Meier survival curve (abbreviated as K-M curves in this work), and the differences were evaluated by the log-rank test. The covariates were selected based on the results of univariate analyses. Multivariate Cox proportional hazards models (abbreviated Cox models in this work) were used to find independent prognostic indicators. We further calculated the Akaike information criterion (AIC) value based on Cox models to compare the prognostic value among variables. The smaller the AIC value is, the better the fit. Clinicopathological characteristics of the three histological subtypes were assessed through the χ2 test or Fisher’s exact test. Continuous variables were translated to categorical variables. Statistical analyses were conducted with SPSS software (version 25.0, SPSS). A P value<0.05 (two-sided) was considered statistically significant.
Clinico-pathological characteristics of different histological subtypes
Among all CRC patients, there were 84.3% (3,246/3,852) patients in the AC group, 7.3% (280/3,852) patients in the AMC group, and 8.5% (326/3,852) patients in the MAC group. Each subtype showed unique clinic-pathological characteristics.
Tumors with a mucinous history (AMC and MAC) tended to be found in the proximal colon (11.6% in AC vs. 28.2% in AMC vs. 30.7% in MAC) and at a later TNM stage (44.2% in AC vs. 48.9% in AMC vs. 50.3% in MAC) when diagnosed; moreover, they were more likely to have higher serum CEA levels (46.9% in AC vs. 56.1% in AMC vs. 53.4% in MAC), higher Ki-67 protein levels (53.6% in AC vs. 47.9% in AMC vs. 98.5% in MAC), and larger lesion sizes (78.7% in AC vs. 89.3% in AMC vs. 89.9% in MAC) (Table 1).
| Factors | n (%) | P (AC vs. AMC) | P (AC vs. MAC) | P (AMC vs. MAC) | ||
|---|---|---|---|---|---|---|
| AC (n=3298) | AMC (n=286) | MA (n=337) | ||||
| Age | 0.229 | 0.089 | 0.773 | |||
| ≥60 | 2008 (61.9) | 163 (58.2) | 186 (57.1) | |||
| Sex | 0.66 | 0.367 | 0.761 | |||
| Male | 2083 (64.2) | 176 (62.9) | 201 (61.7) | |||
| BMI | 0.506 | 0.665 | 0.412 | |||
| ≥28 | 440 (13.6) | 34 (12.1) | 47 (14.4) | |||
| HP | 0.237 | 0.11 | 0.806 | |||
| Presence | 943 (29.1) | 72 (25.7) | 81 (24.8) | |||
| CHD | 0.43 | 0.092 | 0.062 | |||
| Presence | 323 (10.0) | 32 (11.4) | 23 (7.1) | |||
| DM | 0.704 | 0.338 | 0.683 | |||
| Presence | 408 (12.6) | 33 (11.8) | 35 (10.7) | |||
| Smoking history | 0.012 | 0.001 | 0.63 | |||
| Presence | 2111 (65.0) | 203 (72.5) | 242 (74.2) | |||
| Drinking history | 0.121 | 0.002 | 0.291 | |||
| Presence | 2256 (69.5) | 207 (73.9) | 253 (77.6) | |||
Family history of |
0.918 | 0.979 | 0.922 | |||
| Presence | 506 (15.6) | 43 (15.4) | 51 (15.6) | |||
Family history of |
0.094 | 0.168 | 0.023 | |||
| Presence | 347 (10.7) | 21(7.5) | 43 (13.2) | |||
| CEA | 0.003 | 0.025 | 0.506 | |||
| Positive | 1521 (46.9) | 157 (56.1) | 174 (53.4) | |||
| CRP | 0.512 | 0.084 | 0.515 | |||
| Positive | 125 (3.9) | 13 (4.6) | 19 (5.8) | |||
| Tumor position | <0.001 | <0.001 | 0.272 | |||
| Right colon | 376 (11.6) | 79 (28.2) | 100 (30.7) | |||
| Left colon | 640 (19.7) | 42 (15.0) | 61 (18.7) | |||
| Rectum | 2230 (68.7) | 159 (56.8) | 165 (50.6) | |||
| Tumor size | <0.001 | <0.001 | 0.812 | |||
| >20mm | 2556 (78.7) | 250 (89.3) | 293 (89.9) | |||
| Lesion amount | 0.001 | 0.348 | 0.005 | |||
| Unifocal | 3123 (96.2) | 258 (92.1) | 317 (97.2) | |||
| Surgical therapy | 0.003 | 0.338 | 0.11 | |||
| Laparotomy | 1973 (60.8) | 195 (69.9) | 207 (63.5) | |||
| Tumor differentiation grade | 0.085 | 0.902 | 0.176 | |||
| Undifferentiation | 426 (13.1) | 47 (16.8) | 42 (12.9) | |||
| Ki-67 protein level | 0.064 | <0.001 | <0.001 | |||
| Positive | 1740 (53.6) | 134 (47.9) | 321 (98.5) | |||
| PNI | 0.145 | <0.001 | 0.023 | |||
| Presence | 1191 (36.7) | 115 (41.1) | 164 (50.3) | |||
| LVI | 0.771 | 0.124 | 0.392 | |||
| Presence | 924 (28.5) | 82 (29.3) | 106 (32.5) | |||
| TNM stage | 0.008 | 0.044 | 0.446 | |||
| I | 541 (16.7) | 27 (9.6) | 40 (12.3) | |||
| II | 1271 (39.2) | 116 (41.4) | 122 (37.4) | |||
| III | 1434 (44.2) | 137 (48.9) | 164 (50.3) | |||
Note: AC: Adenocarcinoma, AMC: Adenocarcinoma with Mucinous Composition, MAC: Mucinous Adenocarcinoma, BMI: Body Mass Index, HP: Hypertension, CHD: Chronic Heart Disease, DM: Diabetes Mellitus, CEA: Carcinoembryonic Antigen, CRP: C-Reactive Protein, PNI: Perineural Invasion, LVI: Lymphvascular Invasion, TNM: Tumor Node Metastasis
Table 1: Clinicopathological characteristics of patients with stage I-III colorectal adenocarcinoma, adenocarcinoma with mucinous composition, and mucinous adenocarcinoma.
Distinguishments were also shown between AMC and MAC, although their similarities in clinicopathological characteristics were described above. The Ki-67 protein level of MAC was higher than that of AMC (47.9% in AMC vs. 98.5% in MAC); furthermore, MAC tended to be unifocal lesions (69.9% in AMC vs. 97.2% in MAC) (Table 1).
Median follow-up time and number of cases
Taking OS as the endpoint, the median follow-up among surviving patients was 51 months in the AC group, 46 months in the AMC group, and 41 months in the MAC group. When using DFS as the endpoint, the median follow-up time among the surviving patients was 53 months in the AC group, 52 months in the AMC group, and 51 months in the MAC group. The number of patients lost to follow-up was 109 (109/3,852, 2.8%).
At the 5-year follow-up visit, 589 in the AC group, 72 patients in the AMC group, and 165 in the MAC group had died. Regarding tumor recurrence/distant metastasis, 669 in the AC group, 68 patients in the AMC group, and 124 in the MAC group had the case at the 5-year follow-up visit.
Prognostic implication of mucinous histology
Mucinous content can predict OS and DFS. The mean OS times and OS rates of the three groups differed significantly in the logrank test (Table 2 and Figure 2). The mean DFS times and DFS rates between AC and MAC and between AMC and MAC also differed significantly. Significant prognostic discrimination among groups was confirmed. MACs had the worst oncological outcomes (Figure 3).
| Histological type | Overall survival (mean ± SD) | Disease-free survival (mean ± SD) |
|---|---|---|
| AC | 156.003 ± 4.184 | 148.452 ± 6.754 |
| AMC | 75.069 ± 2.654 | 81.117 ± 2.483 |
| MA | 61.672 ± 5.275 | 95.779 ± 4.729 |
Note: SD: Standard Deviation, AC: Adenocarcinoma, AMC: Adenocarcinoma with Mucinous Composition, MA: Mucinous Adenocarcinoma
Table 2: Survival time of stage I-III colorectal adenocarcinoma, adenocarcinoma with mucinous composition, and mucinous adenocarcinoma.
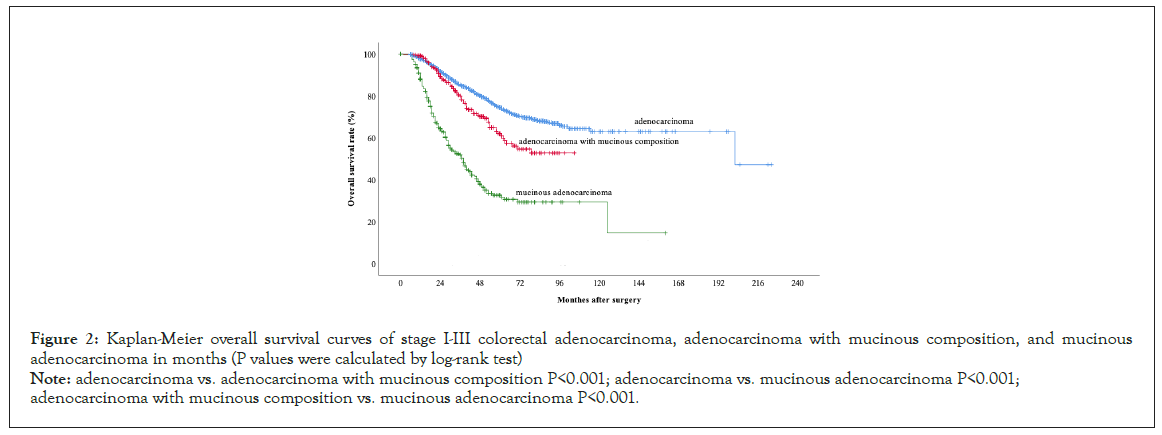
Figure 2:Kaplan-Meier overall survival curves of stage I-III colorectal adenocarcinoma, adenocarcinoma with mucinous composition, and mucinous adenocarcinoma in months (P values were calculated by log-rank test)
Note: adenocarcinoma vs. adenocarcinoma with mucinous composition P<0.001; adenocarcinoma vs. mucinous adenocarcinoma P<0.001; adenocarcinoma with mucinous composition vs. mucinous adenocarcinoma P<0.001.
Figure 3: Kaplan-Meier disease-free survival curves of stage I-III colorectal adenocarcinoma, adenocarcinoma with mucinous composition, and mucinous
adenocarcinoma in months (P values were calculated by log-rank test).
Note: adenocarcinoma vs. adenocarcinoma with mucinous composition P=0.277; adenocarcinoma vs. mucinous adenocarcinoma P<0.001; adenocarcinoma
with mucinous composition vs. mucinous adenocarcinoma P<0.001.
The implications of other potential prognostic predictors were also examined. For OS, serum CEA level (crude hazard ratio HR=1.474; 95% confidence interval CI=1.293, 1.681; P<0.001), serum CRP level (crude HR=1.566; 95% CI=1.152, 2.128; P=0.004), tumor position (crude HR Rectum=0.786; 95% CI Rectum=0.657, 0.940; P Rectum=0.009; P Total=0.030), tumor size (crude HR=1.481; 95% CI=1.235, 1.775; P<0.001), surgical therapy (crude HR=0.756; 95% CI=0.653, 0.874; P<0.001), tumor differentiation grade (crude HR=3.002; 95% CI=2.579, 3.494; P<0.001), Ki-67 protein level (crude HR=0.727; 95% CI=0.637, 0.831; P<0.001), PNI (crude HR=2.145; 95% CI=1.882, 2.446; P<0.001), LVI (crude HR=2.500; 95% CI=1.882, 2.446; P<0.001), and TNM stage (crude HR Stage II=1.753; 95% CI Stage II=1.341, 2.291; P Stage II<0.001; crude HR Stage III=4.024; 95% CI Stage III=3.124, 5.184; P Stage III<0.001; P Total<0.001 ) were the influencing factors (Table 2). Age (crude HR=0.873; 95% CI=0.764, 0.997; P=0.045), HP (crude HR=0.853; 95% CI=0.719, 0.970; P=0.019), serum CEA level (crude HR=1.561; 95% CI=1.367, 1.783; P<0.001), tumor size (crude HR=1.331; 95% CI=1.113, 1.590; P=0.002); lesion mount (crude HR=0.658; 95% CI=0.492, 0.880; P=0.005), tumor differentiation grade (crude HR=2.254; 95% CI=1.926, 2.639; P<0.001), Ki-67 protein level (crude HR=1.341; 95% CI=1.171, 1.535; P<0.001), PNI (crude HR=2.126; 95% CI=1.863, 2.425; P<0.001), LVI (crude HR=2.387; 95% CI=2.092, 2.724; P<0.001), and TNM stage (crude HR Stage II=2.108; 95% CI Stage II=1.556, 2.857; P Stage II<0.001; crude HR Stage III=5.288; 95% CI Stage III=3.962, 7.058; P Stage III<0.001; P Total<0.001) could predict DFS ( Table 3).
| Factors | Overall survival | Disease-free survival | ||||
|---|---|---|---|---|---|---|
| crude HR (95% CI) | P | AIC | crude HR (95% CI) | P | AIC | |
| Histological type | <0.001 | 13797.8 | <0.001 | 14052.42 | ||
| AC | Reference | Reference | ||||
| AMC | 1.537 (1.217, 1.941) | <0.001 | 0.449 (1.143, 0.891) | 0.291 | ||
| MAC | 4.210 (3.551, 4.992) | <0.001 | 2.226 (1.840, 2.693) | <0.001 | ||
| Age | 0.197 | 14004.64 | 0.045 | 14104.73 | ||
| <60 | Reference | Reference | ||||
| ≥60 | 1.092 (0.955, 1.249) | 0.873 (0.764, 0.997) | ||||
| Sex | 0.611 | 14006.06 | 0.303 | 14107.65 | ||
| Female | Reference | Reference | ||||
| Male | 1.036 (0.905, 1.185) | 1.075 (0.937, 1.233) | ||||
| BMI | 0.237 | 14004.96 | 0.863 | 14108.69 | ||
| <28 | Reference | Reference | ||||
| ≥ 28 | 1.117 (0.930, 1.343) | 0.983 (0.811, 1.192) | ||||
| HP | 0.201 | 14004.66 | 0.019 | 14103.03 | ||
| Absence | Reference | Reference | ||||
| Presence | 0.910 (0.787, 1.052) | 0.853 (0.719, 0.970) | ||||
| CHD | 0.339 | 14005.38 | 0.176 | 14106.81 | ||
| Absence | Reference | Reference | ||||
| Presence | 1.113 (0.894, 1.387) | 1.172 (0.931, 1.474) | ||||
| DM | 0.528 | 14005.93 | 0.718 | 14108.59 | ||
| Absence | Reference | Reference | ||||
| Presence | 1.064 (0.878, 1.288) | 1.037 (0.852, 1.261) | ||||
| Smoking history | 0.134 | 14004.05 | 0.374 | 14107.92 | ||
| Absence | Reference | Reference | ||||
| Presence | 1.113 (0.967, 1.280) | 1.066 (0.926, 1.226) | ||||
| Drinking history | 0.404 | 14005.62 | 0.983 | 14108.72 | ||
| Absence | Reference | Reference | ||||
| Presence | 1.063 (0.921, 1.228) | 1.002 (0.868, 1.156) | ||||
| Family history of tumors | 0.159 | 14004.27 | 0.533 | 14108.32 | ||
| Absence | Reference | Reference | ||||
| Presence | 1.145 (0.949, 1.382) | 0.943 (0.784, 1.134) | ||||
| Family history of gastrointestinal tumors | 0.131 | 14003.93 | 0.39 | 14107.96 | ||
| Absence | Reference | Reference | ||||
| Presence | 1.188 (0.950, 1.485) | 0.908 (0.730, 1.131) | ||||
| CEA | <0.001 | 13972.26 | <0.001 | 14064.9 | ||
| Negative | Reference | Reference | ||||
| Positive | 1.474 (1.293, 1.681) | 1.561 (1.367, 1.783) | ||||
| CRP | 0.004 | 13999.14 | 0.251 | 14107.48 | ||
| Negative | Reference | Reference | ||||
| Positive | 1.566 (1.152, 2.128) | 1.210 (0.874, 1.675) | ||||
| Tumor position | 0.03 | 13999.61 | 0.269 | 14106.15 | ||
| Right colon | Reference | Reference | ||||
| Left colon | 0.846 (0.628, 1.050) | 0.129 | 1.106 (0.884, 1.383) | 0.377 | ||
| Rectum | 0.786 (0.657, 0.940) | 0.009 | 0.966 (0.798, 1.169) | 0.72 | ||
| Tumor size | <0.001 | 13986.63 | 0.002 | 14098.23 | ||
| < 20 mm | Reference | Reference | ||||
| ≥ 20 mm | 1.481 (1.235, 1.775) | 1.331 (1.113, 1.590) | ||||
| Lesion amount | 0.137 | 14004.25 | 0.005 | 14101.68 | ||
| Multifocal | Reference | Reference | ||||
| Unifocal | 1.265 (0.928, 1.724) | 0.658 (0.492, 0.880) | ||||
| Surgical therapy | <0.001 | 13991.53 | 0.082 | 14105.65 | ||
| Laparotomy | Reference | Reference | ||||
| Laparostomy | 0.756 (0.653, 0.874) | 1.132 (0.984, 1.302) | ||||
| Tumor differentiation grade | <0.001 | 13842.02 | <0.001 | 14021.49 | ||
| Differentiation | Reference | Reference | ||||
| Undifferentiated | 3.002 (2.579, 3.494) | 2.254 (1.926, 2.639) | ||||
| Ki-67 protein level | <0.001 | 13984.37 | <0.001 | 14090.39 | ||
| Positive | Reference | Reference | ||||
| Negative | 0.727 (0.637, 0.831) | 1.341 (1.171, 1.535) | ||||
| PNI | <0.001 | 13879.13 | <0.001 | 13984.98 | ||
| Absence | Reference | Reference | ||||
| Presence | 2.145 (1.882, 2.446) | 2.126 (1.863, 2.425) | ||||
| LVI | <0.001 | 13831.06 | <0.001 | 13951.61 | ||
| Absence | Reference | Reference | ||||
| Presence | 2.500 (2.192, 2.851) | 2.387 (2.092, 2.724) | ||||
| TNM stage | <0.001 | 13779.55 | <0.001 | 13828.72 | ||
| I | Reference | Reference | ||||
| II | 1.753 (1.341, 2.291) | <0.001 | 2.108 (1.556, 2.857) | <0.001 | ||
| III | 4.024 (3.124, 5.184) | <0.001 | 5.288 (3.962, 7.058) | <0.001 | ||
Note: HR: Hazard Ratio, CI: Confidence Interval, AIC: Akaike Information Criterion, AC: Adenocarcinoma, AMC: Adenocarcinoma with Mucinous Composition, MAC: Mucinous Adenocarcinoma, BMI: Body Mass Index, HP: Hypertension, CHD: Chronic Heart Disease, DM: Diabetes Mellitus, CEA: Carcinoembryonic Antigen, CRP: C-Reactive Protein, PNI: Perineural Invasion, LVI: Lymphvascular Invasion, TNM: Tumor Node Metastasis
Table 3: Univariate analyses and Akaike information criterion value calculation of the prognostic parameters of patients with stage I-III colorectal cancer.
Independent prognostic implication of mucinous histology
Mucinous content is an independent predictor of negative prognoses. MAC (adjusted HR=4.142; 95% CI=3.414, 4.981; P=0.013) and AMC (adjusted HR=1.351; 95% CI=1.065, 1.713; P<0.001) had a significantly higher risk of death than AC. In addition, a significantly higher risk of tumor recurrence/distant metastasis than AC was also shown in MAC (adjusted HR=1.968; 95% CI=1.603, 2.415; P<0.001).
We also used the multivariate Cox model to find other independently associated factors. Age (adjusted HR=1.321; 95% CI=1.153, 1.514; P<0.001), serum CEA level (adjusted HR=1.319; 95% CI=1.153, 1.5141; P<0.001), tumor differentiation grade (adjusted HR=2.508; 95% CI=2.142, 2.936; P<0.001), PNI (adjusted HR=1.441; 95% CI=1.255, 1.654; P<0.001), LVI (adjusted HR=1.595; 95% CI=1.374, 1.852; P<0.001), and TNM stage (adjusted HR Stage III=2.431; 95% CI Stage III=1.842, 3.207; P Stage III<0.001; P Total<0.001) independently implicated OS (Table 4). Independent negative predictor of DFS were serum CEA level (adjusted HR=1.358; 95% CI=1.187, 1.555; P<0.001), tumor differentiation grade (adjusted HR=1.803; 95% CI=1.531, 2.124; P<0.001), Ki-67 protein level (adjusted HR=1.181; 95% CI=1.021, 1.366; P=0.025), PNI (adjusted HR=1.448; 95% CI=1.262, 1.663; P<0.001), LVI (adjusted HR=1.450; 95% CI=1.250, 1.683; P<0.001), and TNM stage (adjusted HR Stage II=1.675; 95% CI Stage II=1.220, 2.300; P Stage II<0.001; adjusted HR Stage III=3.312; 95% CI Stage III=2.432, 4.509; P Stage III<0.001; P Total<0.001) (Table 4).
| Factors | Overall survival | Disease-free survival | ||
|---|---|---|---|---|
| adjusted HR (95% CI) | P | adjusted HR (95% CI) | P | |
| Histological type | <0.001 | <0.001 | ||
| AC | Reference | Reference | ||
| AMC | 1.351 (1.065, 1.713) | <0.001 | 1.022 (0.794, 1.316) | 0.865 |
| MAC | 4.124 (3.414, 4.981) | 0.013 | 1.968 (1.603, 2.415) | <0.001 |
| Age | <0.001 | 0.383 | ||
| <60 | Reference | Reference | ||
| ≥ 60 | 1.321 (1.153, 1.514) | 0.942 (0.825, 1.077) | ||
| CEA | <0.001 | <0.001 | ||
| Negative | Reference | Reference | ||
| Positive | 1.319 (1.153, 1.514) | 1.358 (1.187, 1.555) | ||
| Tumor position | 0.87 | 0.163 | ||
| Right colon | Reference | Reference | ||
| Left colon | 0.980 (0.787, 1.221) | 0.856 | 1.245 (0.991, 1.565) | 0.06 |
| Rectum | 1.024 (0.850, 1.233) | 0.804 | 1.168 (0.958, 1.424) | 0.125 |
| Tumor size | 0.117 | 0.577 | ||
| <20 mm | Reference | Reference | ||
| ≥ 20 mm | 1.164 (0.962, 1.408) | 1.054 (0.876, 1.269) | ||
| Tumor differentiation grade | <0.001 | <0.001 | ||
| Differentiation | Reference | Reference | ||
| Undifferentiated | 2.508 (2.142, 2.936) | 1.803 (1.531, 2.124) | ||
| Ki-67 protein level | 0.79 | 0.025 | ||
| Positive | Reference | Reference | ||
| Negative | 1.020 (0.880, 1.183) | 1.181 (1.021, 1.366) | ||
| PNI | <0.001 | <0.001 | ||
| Absence | Reference | Reference | ||
| Presence | 1.441 (1.255, 1.654) | 1.448 (1.262, 1.663) | ||
| LVI | <0.001 | <0.001 | ||
| Absence | Reference | Reference | ||
| Presence | 1.595 (1.374, 1.852) | 1.450 (1.250, 1.683) | ||
| TNM stage | <0.001 | <0.001 | ||
| I | Reference | Reference | ||
| II | 1.341 (1.010, 1.780) | 0.042 | 1.675 (1.220, 2.300) | <0.001 |
| III | 2.431 (1.842, 3.207) | <0.001 | 3.312 (2.432, 4.509) | <0.001 |
Note: HR: Hazard Ratio, CI: Confidence Interval, AC: Adenocarcinoma, AMC: Adenocarcinoma with Mucinous Composition, MAC: Mucinous Adenocarcinoma, CEA: Carcinoembryonic Antigen, PNI: Perineural Invasion, LVI: Lymphvascular Invasion, TNM: Tumor Node Metastasis
Table 4: Multivariate analyses of the prognostic parameters of patients with stage I-III colorectal cancer.
Fit comparison
Mucinous content was the best predictor for OS after TNM stage. The AIC values of mucinous content and TNM stage were 13,797.801 and 13,779.547, respectively. Regarding DFS, mucinous content was the fifth predictor (AIC=14052.415), while TNM stage was still the best predictor (AIC=13828.719).
Mucinous content is a vital prognostic parameter when selecting management strategies for CRC patients in the clinic. It can independently implicate negative oncological outcomes; moreover, its predictive value was high, ranking second for OS and fifth for DFS. Its high group availability for CRCs in clinicopathological characteristics was also shown in our retrospective cohort investigation. Therefore, we recommend routinely identifying and reporting mucinous content in CRCs, if possible, the specific percentage of mucinous areas. We applied detailed, multifocused, and multivalidated comparisons to refine our work. Fit comparisons based on the AIC value were novel. Furthermore, to the best of our knowledge, this study has the largest sample size among similar investigations in China. Large samples could reduce bias, reflect the real trends, and increase the confidence of the results. This work included many clinically available features, challenged the risk stratification and forecast models that are mainly based on TNM staging, and shed new light on the diagnosis and treatment planning of patients with CRC. Additionally, we pointed out the importance of using multidisciplinary teams (MDTs).
The differences between the left and right colon were the prominent theme of the 2016 ASCO conference [34]. We found that MACs were more common in the proximal colon, similar to previous studies [35], while an analysis based on the SEER dataset in the US reported that MAC was more common in the left colon. Activation of MSI was more common in the right colon, which is the main cause of genetic CRCs such as Lynch syndrome. Poor prognoses of right colon cancer (CC) were also widely found. At the same time, high MSI activation [36-38] and poor prognoses are also characteristics of mucinous history (AMC and MAC). Therefore, further studies are needed to investigate whether different histology are related to the development of right and leftsided CCs. Clinicians tend to adapt adjuvant chemoradiotherapy to patients with stage III CRCs or high-risk stage II CRCs. In our work and previous studies, a mucinous history (AMC and MAC) tended to be diagnosed in advancing TNM stages [39]. This suggested that mucinous content may be an indicator of adjuvant therapy. There were no significant differences in PNI and LVI among the three histological types, and PNI and LVI might not be the prompting indicators for the formation of mucinous content. Drugs that inhibit tumor angiogenesis and tumor neurological metastasis might not be excellent targeted choices, while changes in serum CEA levels provide new ideas. Although mucinous histology is a pathological factor, its correlation with many clinical factors suggests that its discussion at multidisciplinary team meetings (MDTs) might prevent improper clinical decisions in patient management. The exchange of information among disciplines facilitates patient management.
The distinguishing carcinogenic mechanisms of mucinous histology (AMC and MAC) have been widely shown [40, 41], and the effects of mucinous content on treatment response [42-45] and survival have been extensively found. Neglecting mucinous histology might lead to undertreatments. However, TNM stage is still the primary reference in the clinic. Mucinous history is not taken as a predictor of negative outcomes in the American Joint Committee (AJCC) guidelines [3] and the National Comprehensive Cancer Network (NCCN) guidelines [46-48]. AMCs and MACs are often vaguely classified as ACs and adopt similar management methods, although these therapies might be insufficient for both [49]. Giving aggressive tumors moderate approaches would result in earlier tumor recurrence/distant metastasis, and even earlier patient death. This could be validated by the large differences (up to 134 months) in survival time between mucinous history (AMC and MAC) and AC that we obtained from the K-M survival curves; moreover, log-rank tests demonstrated the statistical significance of the differences. Therefore, simply applying the regimens of ACs to all CRCs is not appropriate. In other words, it is necessary to distinguish the three subtypes based on the mucinous content in the clinic. Mucinous histology is a vital reference for formulating patient management plans. Tumors with mucinous content should be treated more thoroughly; additionally, closer follow-up is also appropriate, although there were no strong associations between the intensity of detection and tumor recurrence and patient death.
To further examine our assumption that mucinous content is a great reference to manage CRC patients, it is necessary to use multivariate analyses to minimize the biases of confounding factors and show the independent effects of mucinous content. Moreover, when comprehensive considerations of tumors’ histopathological characteristics were incorporated into clinical care, analyses of tumor subgroups and prognostic interactions among confounders and variables became increasingly important. Multivariate Cox models showed that mucinous histology (AMC and MAC) had independent effects on patient outcomes, tending to have negative outcomes. In addition, the adjusted HRs were large, which suggested that the prognoses of different mucinous contents were indeed different and had high discriminating value. We also used the AIC values obtained from the Cox regression models to compare the prognostic value among variables as multiple validations. The results showed that the predictive value of mucinous content for OS and DFS was excellent. It outperformed many other indicators, although the worthy was slightly inferior to TNM stage. This again highlights the vital role of a mucinous history in the multimodal management of CRC patients, although further studies are needed [50].
The development of tumors is not a single factor, and the other covariates are also worthy of investigation, especially the factors correlated with histological subtypes [51]. Primary tumor laterality still does not serve as a routine reference when selecting adjuvant or palliative care for CRC patients, although its predictive value for oncological outcomes has been found. Our work also obtained negative results when using it as a prognostic index. The predictive value of tumor location for OS disappeared when confounding factors such as mucinous content was excluded. TNM stage, as a widely accepted reference when selecting management methods, performed well in our analyses, while the reasons behind its survival paradoxes still need further elucidation. PNI and LVI also had independent prognostic effects. Age has been previously identified as a risk factor for OS in CRC patients [52,53]. This study supports this notion; our multivariate analyses showed that old age was independently associated with more frequent tumor recurrence/distant metastasis and earlier patient death. However, given the association of old age with treatment complications, the benefits and risks should be carefully traded off when opt methods. Moreover, despite careful screening at the time of patient inclusion, the confounding of no cancer-specific deaths remains unavoidable and requires further elucidation. Although MACs were previously routinely identified as poorly differentiated, recent WHO guidelines suggest that the level of epithelial maturation determines the differentiation and microsatellite instability of MACs, and their histological grade should be carefully considered. Nonetheless, accurate grading criteria have not been provided, and the prognostic value of histological grading in MACs remains unclear. However, in this study, tumor differentiation grade was an independent predictor of negative prognoses.
Our work systematically demonstrated the high prognostic and classification value of mucinous content for CRCs and highlighted its reliability as a clinical reference. Covariates were also cautiously selected and analyzed. However, tumor response to auxiliary examination also needs to be considered when deciding treatment options. MRI could identify mucinous content more accurately than the other imaging modalities and was even more accurate than preoperative biopsies. It shows mucinous hyperintensity on T2-weighted images [12]. In contrast, PET/CT, a commonly used tumor detection method, is not as sensitive [54]. Certainly, this requires further investigation and assistance from other relevant departments.
The advantages of this work included a large sample size, relatively adequate observation period, subgroup analyses and multivalidations, while limitations should also be explained. First, this study has limitations common in retrospective and singlecenter studies. However, our large sample size allowed detailed comparison of baseline characteristics and prognoses among the three subtypes; additionally, the study population was well characterized, and obtained through the detailed CRC registration. Therefore, we deemed that the statistical strength and the results indicated are adequate. Second, estimating mucinous content by visual inspection was generally considered to be less accurate than more precise quantification methods, such as software analysis. However, in our experience, visual inspection remained the most common quantification method in daily practice. Therefore, it was still most translatable to routine pathological assessments. Furthermore, even with specialized software, it is nearly impossible to determine the "true" percentages of the above parameters, because most CRCs in this work were not fully submitted for microscopy, and mucinous content varies from slice to slice within a tumor. Finally, molecular events and genomic features were not examined by us, and more detailed epidemiological factors might also influence the prognostic sensitivity of mucinous content. Nonetheless, the histological subtype of CRCs was an available prognostic factor. Although we did not investigate the differences in molecular mechanisms, AC, AMC and MAC are distinct disease entities, given the discrimination of clinicopathological features and prognoses.
Mucinous content is an independent prognostic parameter for patients with I-III stage colorectal cancer, which should be taken into account for treatment strategy decisions. The exact mechanisms underlying the poor prognoses of mucinous histology should be elucidated in future studies to improve patient management. Furthermore, investigations that analyze the role of mucinous histology as a prognostic marker in no high-risk stage II CRC patients might help identify patients who would benefit from adjuvant chemoradiotherapies. In the near future, mucinous content might play a vital role in tailoring treatment regimens to individual patient characteristics. Whether CRC patients require further classifications based on the percentage of mucinous component should be examined as well.
Ethics approval and consent to participate
This research study was conducted retrospectively from data obtained for clinical purposes and was reviewed and approved by the Ethics Committee of the Affiliate Hospital of Qingdao University (reference number: QYFY WZLL26486). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. The need for written informed consent was waived by the Ethics Committee of the Affiliate Hospital of Qingdao University due to retrospective nature of the study.
Not applicable.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
The authors have no relevant financial or non-financial interests to disclose.
The research was supported by the National Natural Science Foundation of China (Grant number 81802777).
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Xiaolin Ji, Hongsheng Ji and Tao Mao. The first draft of the manuscript was written by Xiaolin Ji and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The research was supported by the National Natural Science Foundation of China (Grant number 81802777).
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
Citation: Tian Z, Ji X, Ji H, Mao T, Li X, Ren M, et al. (2022) Mucinous Content is an Independent Prognostic Parameter for Patients with Stage I-III Colorectal Cancer: A Retrospective Study. Immunogenet Open Access.7:176.
Received: 22-Jul-2022, Manuscript No. IGOA-22-18544; Editor assigned: 25-Jul-2022, Pre QC No. IGOA-22-18544 (PQ); Reviewed: 08-Aug-2022, QC No. IGOA-22-18544; Revised: 15-Aug-2022, Manuscript No. IGOA-22-18544 (R); Published: 22-Aug-2022 , DOI: 10.35248/IGOA.22.7.176
Copyright: © 2022 Tian Z, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.