Journal of Cancer Science and Research
Open Access
ISSN: 2576-1447
ISSN: 2576-1447
Research Article - (2018) Volume 0, Issue 0
Keywords: Multi drug resistance; Colon cancer; Ursolic acid; Caffeine; Nanoparticle
Although cancer cells are inherently more vulnerable to chemotherapy than the majority of normal cells, most anti-cancer drugs are non-selective and can cause injury to normal tissues. Efforts are now focused on attempts to kill cancer cells by more specific targeting while sparing normal cells [1-3]. Nanoparticles can target tumors by a passive or active process. Passive targeting implies that nanoparticles smaller than the fenestrations of endothelial cells can enter the interstitium and be entrapped in the tumour. The combination of leaky vasculature and poor lymphatic drainage results in the well-known Enhanced Permeability and Retention (EPR) effect [4]. Active targeting involves drug delivery to a specific site based on molecular recognition. One approach is to couple a ligand to nanoparticles which can interact with its receptor at the target cell site [5,6].
Anticancer drugs, even if they are located in the tumoralinterstitium can turn out to have limited efficacy against numerous solid tumor types, especially because cancer cells are able to develop mechanisms of resistance and evade chemotherapy [7]. The multidrug resistance (MDR) phenotype, mainly due to expression of the MDR gene family encoding for the membrane proteins called P-glycoprotein (P-gp) represents an important problem in chemotherapy [8]. MDR to anticancer agents remains a major hurdle to successful cancer chemotherapy. The development of effective therapies overcoming MDR against cancer and particularly highly metastatic disease still remains a high priority. Nanoparticles could reduce the MDR that characterizes many anti-cancer drugs by a mechanism of internalization of the drug [9]. To reduce the toxicity and increase the therapeutic efficacy of anticancer drugs, various drug carriers such as soluble polymers, liposomes and self-assembled nanoparticles have been investigated [10-12].
PLGA poly (lactic-co-glycolic acid) is one of the most successfully used biodegradable Nano systems for the development of Nano medicines because it undergoes hydrolysis in the body to produce the biodegradable metabolite monomers, lactic acid and glycolic acid [13]. PLGA have potential because of their size, hydrophobic core with hydrophilic periphery, and biocompatibility [14]. Ursolic acid (UA), a pentacyclictriterpenoid carboxylic acid found in plants, has various biological properties, including anti-inflammatory, anticancer, antiangiogenic, and antioxidative activities [15,16]. Numerous reports of UA’s in vitro activities against tumor cell lines have appeared in the literature [17] and the possible mechanisms of action have been reviewed recently [18]. Caffeine (1, 3, 7-trimethylxanthine) is found in both coffee and tea, so a great number of people are exposed to various doses of caffeine. It acts as a stimulant for the central nervous, respiratory and cardiac system. Caffeine significantly reduces cancer risk caused by environmental and dietary carcinogens [19] and the protective action of caffeine against a variety of chemical carcinogens was established by several studies, carried out by Abraham [20].
Materials
Poly (lactic-co-glycolic acid) (PLGA) 65:35 (MW 40,000-75,000), poly vinyl alcohol (PVA) (MW 25,000), Ursolic acid (UA), Caffeine (Caf), thiobarbituric acid (TBA), phenazinemethosulphate (PMS), nitrobluetetrazolium (NBT), 5, 5-dithiobis 2-nitrobenzoic acid (DTNB), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), ethidium bromide (EtBr), acridine orange (AO), fetal calf serum (FCS), fetal bovine serum (FBS), phosphate buffer saline (PBS), DMEM medium, glutamine- penicillin-streptomycin solution, ficoll-histopaque 1077, trypsin-EDTA were purchased from Sigma Chemicals Co., St. Louis, USA.
Nanoparticle preparation
UA-PLGA nanoparticles and Caf-PLGA nanoparticles were prepared by nanoprecitation method as previously described by Peltonen [21]. 0.1% of PVA was prepared by dissolving 100 mg of PVA in 100 ml distilled water in magnetic stirrer at 60ºC. Organic solution of PLGA (100 mg) and UA/Caf (10 mg) in acetone (10 ml) was added to PVA solution (10 ml). The sample was sonicated at 25 watts for 2 min (Sonics VC-130, Sonics and Materials Inc. CT, USA) and kept the sample under magnetic stirrer at room temperature for 6 h. To remove the non-incorporated drug, the obtained Nano suspension was centrifuged and washed with distilled water twice at 14,000 rpm for 30 min. The supernatant containing the free drug was discarded and the pellet was freeze dried at -50 ºC.
Drug encapsulation efficiency
Drug encapsulation efficiency was described by the method of Mathew [22]. Drug loaded nanoparticles were centrifuged at 14,000 rpm for 30 min. 3 ml of the supernatant obtained during the centrifugation was taken in a cuvette and the absorbance value at 266 nm was recorded with a UV spectrophotometer.

Surface morphology of the nanoparticles
The surface morphology of UA-PLGA nanoparticles and Caf-PLGA nanoparticles were characterized using the following.
Nanoparticle size and surface charge: DLS (Zetasizer Nano, Malvern Instruments Ltd. United Kingdom) was used to measure the average size and size distribution of the prepared nanoparticles. Three different batches were analyzed to give an average value and standard deviation for the particle diameter and zeta potential.
Transmission electron microscopy: A transmission electron microscope (CEM 902A; Zeiss, Germany) was used to observe the twodimensional, relative size morphology of drug loaded nanoparticles. Nanoparticle suspension was diluted in deionized water (1:100 vol/vol) and before observation nanoparticles were negatively stained with a solution of 1 % phosphotungstic acid.
X-ray diffraction: The X-ray diffraction (XRD) experiments were carried out using XRD instrument (X’Pert PRO PANalytical). 5 μg of the drug loaded nanoparticles powder was taken in the sample holder and XRD was recorded.
Fourier transform infrared spectroscopy: Fourier transform infrared spectroscopy (FTIR) spectra of drug loaded nanoparticles were recorded using FTIR spectrophotometer (Perkin Elmer Spectrum RXI). The characteristic peaks were recorded for each sample.
In vitro drug release
Drug release profiles of nanoparticles were investigated in PBS and 10 % FBS medium at pH 7.4 accordingly by the method of Dong [23]. Five microgram of lyophilized drug loaded nanoparticles were dispersed in 30 ml of 10% FBS/PBS and placed in water bath shaker set at 37°C with a shaking speed of 120 rpm. At 1h time intervals 3 ml of supernatant from the sample was taken for analysis and the same amount of fresh 10% FBS/PBS was replaced to the sample. Each time absorbance value at 266 nm was recorded using UV spectrophotometer.
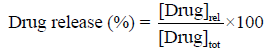
Cell culture
The present work was carried out in colon cancer cell lines
(HT29). HT29 cells were obtained from National Centre for Cell Science (NCCS), Pune, India. The present work has been approved by Institutional Ethical Committee (IEC), Biogenix, Trivandrum. The cells were grown as monolayer in DMEM medium supplemented with 10% FCS, 1mM sodium pyruvate and 10 mM HEPES, 1.5 g/L sodium bicarbonate, 2 mM L-glutamine, and 100 U/ml penicillin-streptomycin at 37°C in 5% CO2 incubator.
Viability of HT29 cells treated with drug loaded nanoparticles: Cells were treated with different Cells were treated with different concentration of UA-PLGA NPs and Caf-PLGA NPs (6.25, 12.5, 25, 50 and 100 μg) and incubated for 24 h at 5% CO2 incubator. Cytotoxicity was observed by MTT assay by the method of Mosmann, 1983 [24]. IC50 was calculated by (ED50plus software V 1.0) and the parameter was repeated for six times. IC50 values were calculated and the optimum dose was used for further study.
Experimental groups: The HT29 cells were divided into 4 experimental groups. Group 1: Untreated control cells, Group 2: UAPLGA nanoparticles treatment alone (88.10 μg), Group 3: Caf-PLGA nanoparticles treatment alone (49.63 μg) and Group 4: UA-PLGA nanoparticles (88.10 μg) + (after one hour) Caf-PLGA nanoparticles (49.63 μg ) treatment.
Apoptotic morphological changes: Apoptotic morphological changes during drug loaded nanoparticles treatment were analyzed by AO/EtBr staining. This dual staining method differentiates condensed chromatin of dead apoptotic cells from the intact normal cell nuclei [25].
Measurement of lipid peroxidation: At the end of the 24 h incubation period, HT29 cells were scrape harvested in ice-cold PBS and centrifuged at 4°C, the supernatant was discarded, and the cell pellets were disrupted for the measurement of TBARS, according to the procedures described elsewhere Niehaus and Samuelsson [26].
Measurement of reduced glutathione levels: After 24 h incubation, drug loaded nanoparticles treated HT29 cells were used for the measurement of GSH level. The total reduced glutathione (GSH) content was measured by the method of Ellman [27].
Statistical analysis
Statistical analysis was performed by one-way ANOVA followed by DMRT taking p
Preparation and characterization of prepared nanoparticles
UA-PLGA nanoparticles and Caf-PLGA nanoparticles were prepared by nanoprecipitation method. In order to predict the in vitro behavior of the prepared nanoparticles, their mean hydrodynamic diameter, polydispersity index (PDI), zeta potential and morphology were evaluated. DLS results revealed that the average size of the UAPLGA nanoparticle was 120 nm and the PDI was found to be 0.060 and Caf-PLGA nanoparticle was 100 nm and the PDI was found to be 0.054. Zeta potential also varied moderately (−24.8 ± 0.4 mV and −14.3 ± 0.5 mV) with the amount of PVA used. Particles prepared with PVA had less surface charge (Figure 1A and 1B). TEM analysis showed UAPLGA nanoparticles and Caf-PLGA nanoparticles with smooth surface morphology. No breakage or tear was noticed. Figure 2 illustrates the TEM image of UA-PLGA nanoparticles and Caf-PLGA nanoparticles which show the nanoparticles were spherical in shape with the size of 120 nm and 100 nm. The broad peak shows that the formulation of UA-PLGA nanoparticles and Caf-PLGA nanoparticles partially crystalline in nature. The broad peaks observed in the diffractogram at around 2° for UA, 17° for UA-PLGA nanoparticles, 17° for Caf and 12° for Caf-PLGA nanoparticles in the formulation. The crystallinity decreases during preparation was shown in Figure 3A and 3B there is no context to the one described in next statement for diffractogram and the statements are not in right order. The FTIR band at 3125 cm-1 is due to stretching vibrations of Ar-H, (-CH) several band at 2625 cm-1 (CH), 2 or 3 band of methyl group. The presence of aryl carboxylic group in the region 1700 cm-1 represents C=O stretching vibration. Presence of aryl nitro group in the region of 1525 cm-1 is also observed. Etherial group is found at 1200 cm-1, where C-O-C shows very strong stretching in UA-PLGA nanoparticles and the band at 3510 cm-1 represented O-H group stretching of O-H, H-bonded single bridge. The presence of aryl carboxylic group in the region 1700 cm-1 represents C=O stretching vibration. Presence of aryl nitro group in the region of 1500- 1150 cm-1 is also observed. Etherial group is found at 1150 cm-1, where C-O-C shows very strong stretching for Caf-PLGA nanoparticles shown in Figure 4A and 4B.
Drug encapsulation efficiency
Table 1 shows the amount of UA and Caf encapsulated in PLGA nanoparticles. The encapsulation efficiencies of UA-PLGA nanoparticles are in the range of 69% and Caf-PLGA nanoparticles are in the range of 78%. Encapsulation efficiency was also increased by the addition of PVA. This is due to the protective effect of PVA micelles, which caused the drug to spontaneously accumulate in the PLGA.
| Fabrications variables | Encapsulation Efficiency (%) | Mean Diameter (nm) | Polydispersity Index | Size (nm) | Zeta Potential (mV) | |
| Polymer | Drug | |||||
| PLGA | UA | 69 ± 0.21 | 14.52 ± 80.3 | 0.06 ± 0.11 | 120 ± 4.8 | -24.8 ± 0.4 |
| PLGA | Caf | 78 ± 4.36 | 133.6 ± 39.5 | 0.054 ± 0.09 | 100 ± 4.3 | -14.3 ± 0.5 |
Table 1: Shows the drug encapsulation efficiency, polydispersity index, size and surface charge of the UA-PLGA nanoparticles and Caf-PLGA nanoparticles.
In vitro drug release
Figure 5 shows the percentage drug release in PBS during different time interval. After 3 h incubation, 7% UA was released in the PBS and the maximum of 69% UA release was observed upon 30 h incubation and after 3 h incubation 9% Caf was released in the PBS and the maximum of 82% Caf release was observed upon 30 h incubation.
Dose fixation study
Figure 6 shows the % cytotoxicity of UA-PLGA nanoparticles and Caf-PLGA nanoparticles (6.25, 12.5, 25, 50 and 100 μg) in HT29 cells. Inhibitory concentration 50 (IC50) values were found to be 88.10 μg and 49.63 μg respectively, and it was used for further experiments.
Apoptotic morphology
Figure 7A shows the photomicrographs of apoptotic morphological changes in UA-PLGA nanoparticles, Caf-PLGA nanoparticles and UAPLGA nanoparticles + Caf-PLGA nanoparticles treated cells. The % apoptotic cell death was increased during UA-PLGA nanoparticles, Caf-PLGA nanoparticles and UA-PLGA nanoparticles + Caf-PLGA nanoparticles treatment. It was found that treated cells showed 64%, 87% and 93% of apoptotic cells, respectively (Figure 7B).
Changes in the levels of TBARS and reduced glutathione
The levels of TBARS were significantly increased in UA-PLGA nanoparticles + Caf-PLGA nanoparticles treated cells (Figure 8A). UAPLGA nanoparticles + Caf-PLGA nanoparticles treated cells showed progressively increased levels of TBARS when compared to UA-PLGA nanoparticles and Caf-PLGA nanoparticles treated cells. The levels of GSH were found to be greatly decreased in UA-PLGA nanoparticles + Caf-PLGA nanoparticles treated cells when compared to UA-PLGA nanoparticles and Caf-PLGA nanoparticles treatment alone (Figure 8B).
The main goal of this work was to develop a polymeric delivery system, capable of increasing the therapeutic index of the drug and devoid of adverse effects. The nano precipitation method is recommended for the incorporation of hydrophobic drugs into polymeric nano particles [28,29]. Nevertheless, as described by several authors and also as demonstrated in this work, the establishment of a protocol that allows nanoparticles precipitation, while avoiding extensive diffusion of the drug along with the solvent aiming at obtaining high values of drug encapsulation is a challenging issue [30]. The organic/aqueous phase ratio is among the most critical parameters for the spontaneous formation of colloidal particles by the nano precipitation method [31].
The physicochemical characteristics of colloidal systems, namely size and surface charge are recognized to affect their physical stability and to significantly influence their interaction as well as the release rate of an incorporated substance and their interaction with cells [32,33]. The method used in this work allowed the instantaneous and reproducible formation of nanoparticles exhibiting diameter below 120 nm and low polydispersity indexes, indicating an homogeneous size distribution. As mentioned above, these findings were confirmed by the TEM data which also showed that nano spheres were spherical in shape. Nanoparticles were shown to exhibit a negative surface charge which can be attributed to the type of polymer used and more specifically to the presence of polymeric carboxylic groups on the nanoparticle surface [34,35]. Further we noticed that the PLGA degraded gradually and released the drug in a sustained manner. 7% UA was released in 3h and 69% UA was released in 30 h incubation in the PBS and 9% Caf was released in 3h and 82% Caf was released in 30 h incubation in the PBS. This result indicates that the prepared nanoparticles are useful for controlled delivery system for cancer treatment [36].
Furthermore, we evaluated the anticancer activity of dug loaded nanoparticles in HT29 cell line. It was found that UA-PLGA nanoparticles and Caf-PLGA nanoparticles could greatly inhibit the HT29 cell growth. The reason for increased cytotoxicity observed in the UA-PLGA nanoparticles and Caf-PLGA nanoparticles group might be due to increased cellular uptake and sustained drug delivery. Enhanced cytotoxicity during UA-PLGA nanoparticles and Caf- PLGA nanoparticles treatment indicates that PLGA has the potency to transport more UA and Caf into the cells, thus achieving greater cytotoxicity. IC50 values for UA-PLGA nanoparticles and Caf-PLGA nanoparticles in our study were 88.10 μg and 49.63 μg. Previously 88.69 μM value reported before for PTX [37]
We have observed UA-PLGA nanoparticles +Caf-PLGA nanoparticles pretreatment significantly increased apoptotic morphological changes in HT29 cells than UA-PLGA nanoparticles and Caf-PLGA nanoparticles treatment alone. Apoptosis has been shown to be a significant mode of cell death after cytotoxic drug treatment [38]. We observed significant increase in lipid peroxidation indices in UAPLGA nanoparticles +Caf-PLGA nanoparticles treated cancer cells. Previous report suggests that similar phenolic acid has been reported to stimulate hydroxyl radical formation and reduce ferrylmyoglobin which suggests their potential prooxidant action. The effect of caffeic acid on the Fenton reaction has been documented using the ESR spin trapping technique [39]. The phenol ring-containing compounds are oxidized by peroxidase/ROS to phenoxyl radicals that co oxidize GSH to form a thyl radical (GS), which then reacts with GSH to form a disulfide radical anion. The carboxylic acid group with the adjacent unsaturated C–C double bond can contribute to the stability of the radical via resonance or by providing additional attack sites for free radicals [40]. This might be the reason for decreased GSH levels in UA-PLGA nanoparticles and Caf-PLGA nanoparticles treated NCI-H460 cells. Previous studies have shown that phytochemicals depleted intracellular antioxidants, thereby induced cancer cell death [41].
In the present study we have shown that drug loaded nanoparticles with a high loading efficiency and release. We therefore suggest that UA-PLGA nanoparticles +Caf-PLGA nanoparticles are a robust and attractive drug delivery system for effective chemotherapy against human colon cancer.