Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Case Report - (2020)
Purpose: The aim of this study is to report the case of a patient diagnosed with butterfly pattern dystrophy (BPD), which during yearly follow-up visits developed a concomitant pachychoroidal neovasculopathy (PNV).
Methods: Case report focusing on the role multimodal imaging in patients with pattern dystrophies (PDs)
Case report: We describe a case of a 52-year-old woman,which was initially diagnosed with BPD by fundus examination, fundus autofluorescence and optical coherence tomofraphy findings. Interestingly, during yearly follow-up visits, the presence of a thickened choroid bilaterally associated with the onset of concomitant choroidal neovascularizations (CNVs) in the right eye was made possibile by adopting multimodal imaging, in particular optical coherence tomography angiography. Fluorescein angiography and indocyanine green angiography findings helped us to make the diagnosis of PNV.
Conclusion: We described for the first time a case of PD, which after several years progressed to PNV. Hence, we deem that multimodal imaging and in particular optical coherence tomography angiography represent an important diagnostic tool in the management of PDs.
Retina; Macula; Retinal dystrophy; Pattern dystrophy; OCT; Octa
Pattern dystrophies (PDs) are an heterogenous group of autosomal dominant inherited disorders affecting the retinal pigment epithelium (RPE), including butterfly-type pattern dystrophy (BPD), adult-onset foveomacular vitelliform dystrophy, Sjögren’s reticular-type pattern dsytrophy and fundus pulverulentus [1]. Usually bilateral, PD are often diagnosed during the third or fourth decades in asymptomatic subjects; in particular, while in some cases PDs tend not to interfere with visual acuity, severe vision loss has been reported in more than 50% of the patients aged 70, caused by RPE atrophy or the formation of choroidal neovascularization (CNVs) [2].
Each subtype of PDs is characterized by its typical pattern of RPE modifications; however, PDs are linked by the deposition of extracellular material (such as lipofuscin) under the neuroepithelial retinal layer [3]. In particular, BPD is characterized by a pigment deposition resembling a butterfly wings-like shape in the fovea [4].
In this case report we will discuss the multimodal imaging adopted for the diagnosis and for monitoring the natural history of the disease, including fundus examination, Fundus Autofluorescence (FAF), Fluorescein Angiography (FA), Indocyanine Green Angiography (ICGA), Swept-Source Optical Coherence Tomography (SS-OCT) and Swept-Source Optical Coherence Tomography Angiography (SS-OCTA).
A 52-year-old woman presented at the Retina Center of the University Eye Clinic in Genoa in 2012 for a control visit. Best corrected visual acuity was 20/20 in both eyes. Medical history was unremarkable for systemic diseases or other ocular disorders. Slit-lamp examination of the anterior segment and Intraocular Pressure (IOP) were normal.
Fundus examination revealed the presence of a yellowish deposit at the posterior pole and scattered areas of depigmentation in the macular area, in both eyes. FAF showed the presence of bilateral patchy hyperautofluorescent spots mixed with hypoautofluorescent areas at the macula; Spectral-domain OCT scans (Topcon 3D OCT-2000) revealed the presence of a thickening of the RPE-Bruch’s membrane complex with involvement of the outer retinal layer (Figure 1).
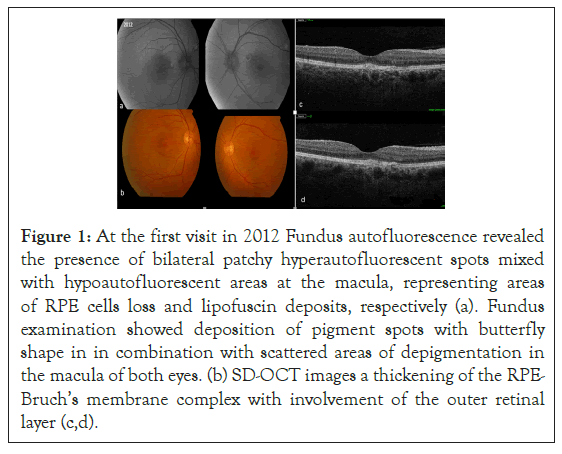
Figure 1: At the first visit in 2012 Fundus autofluorescence revealed the presence of bilateral patchy hyperautofluorescent spots mixed with hypoautofluorescent areas at the macula, representing areas of RPE cells loss and lipofuscin deposits, respectively (a). Fundus examination showed deposition of pigment spots with butterfly shape in in combination with scattered areas of depigmentation in the macula of both eyes. (b) SD-OCT images a thickening of the RPE-Bruch’s membrane complex with involvement of the outer retinal layer (c,d).
Yearly visit performed on 2016, visual acuity of the right eye dropped to 20/25. [5]. The adoption of a SS-OCT (Topcon Triton DRI) machine allowed us to highlight a thick choroid. Furthermore, SS-OCTA detects the presence of a branching network of CNVs growing under the RPE in the patient’s right eye. Hence, the combination of a thick choroid associated with a concomitant type 1 CNVs addressed us to make a new diagnosis of pachychoroidal neovasculopathy (PNV) (Figure 2). OCTA of the left eye did not show any CNV.
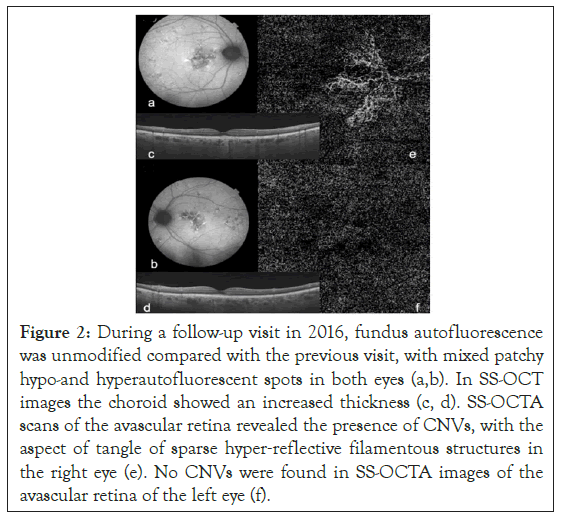
Figure 2: During a follow-up visit in 2016, fundus autofluorescence was unmodified compared with the previous visit, with mixed patchy hypo-and hyperautofluorescent spots in both eyes (a,b). In SS-OCT images the choroid showed an increased thickness (c, d). SS-OCTA scans of the avascular retina revealed the presence of CNVs, with the aspect of tangle of sparse hyper‐reflective filamentous structures in the right eye (e). No CNVs were found in SS-OCTA images of the avascular retina of the left eye (f).
In 2019, during a follow-up visit, visual acuity dropped at 20/32 in both eyes. Fundus examination and FAF showed no significant differences, however in the right eye SS-OCT revealed the presence of a dome-shaped RPE detachment containing patchy hyperreflective material. Left eye remained unchanged. SS-OCTA of right eye, showed the vascular network more enhanced and tortuous as compared with the previous visits. FA revealed an unspecific early hyperfluorescence with moderate leakage/staining in the late phase; however, at the level of the dome-shaped RPE detachment seen at SS-OCT, ICGA revealed the presence of a focal hyperfluorescence surrounded by a late and faint hyperfluorescence resembling the typical neovascular plaque. Those findings suggested the diagnosis of polypoidal choroidal vasculopathy (PCV). Although in the left eye SSOCTA did not showed any CNV, late phase ICGA, revealed a late hyperfluorescence due to hyperpermeability of the choroid (Figure 3).
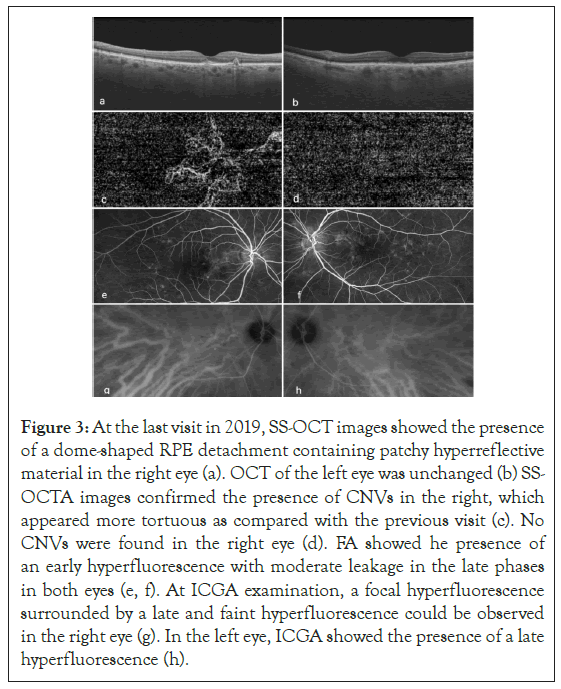
Figure 3: At the last visit in 2019, SS-OCT images showed the presence of a dome-shaped RPE detachment containing patchy hyperreflective material in the right eye (a). OCT of the left eye was unchanged (b) SSOCTA images confirmed the presence of CNVs in the right, which appeared more tortuous as compared with the previous visit (c). No CNVs were found in the right eye (d). FA showed he presence of an early hyperfluorescence with moderate leakage in the late phases in both eyes (e, f). At ICGA examination, a focal hyperfluorescence surrounded by a late and faint hyperfluorescence could be observed in the right eye (g). In the left eye, ICGA showed the presence of a late hyperfluorescence (h).
Pattern Dystrophies (PDs) were first described by Gass as an heterogenous family of autosomal dominant inherited retinal diseases affecting the RPE and leading ultimately to macular atrophy and CNVs onset [1]. It has been demonstrated that mutations occurring in the Retinal Degeneration Slow (RDS)/ peripherin gene, situated on chromosome 6p21.2, are responsible for PDs development [6]. In this regard, this latter gene encodes a 39 kd membrane-associated glycoprotein expressed selectively by cone and rod photoreceptors; hence, this mutation has been shown to cause photoreceptor membrane disruption and lead to the accumulation of lipofuscin and degenerative material at the RPE level [7].
Clinically, in most of the cases PDs lead to a mild-moderate visual loss during the fifth or sixth decades of life; however, it is well documented the possibility of developing macular atrophy or CNVs in a minority of patients, who are consequently affected by severe visual loss [8].
The association between PDs and CNVs onset has been widely reported in literature [9]. In this regard, both photodynamic therapy (PDT) with verteporfin and anti-VEGF agents have been investigated in the management of PDs, with this latter strategy having shown the most promising results in terms of clinical efficacy [10]; however, in some cases CNVs secondary to PDs may resolve spontaneously without need of treatment [11].
The multimodal imaging of our case is partly in line with previous studies investigating this subtype of PD. Fundus examination revealed the presence of a central depigmentation area surrounded by yellowish pigment spots, resembling the shape of butterfly wings; in line with these findings, histopathological evidence has reported RPE and photoreceptor cells layers loss associated with the contemporary lipofuscin accumulation in macular region [7]. In this regard SS-OCT imaging was useful in describing the presence of thinned outer retinal layers (IS/OS) and hyperreflective lipofuscin deposits under the RPE.
Furthermore, as previously reported, FAF examination pointed out the presence of patchy hyper/hypoautofluorescent areas (representing lipofuscin deposition) mixed with areas of RPE loss at the macula [12]. In addition, FA was helpful as well in distinguishing BPD from other subtypes of PDs [13].
However, our case differs in some aspects from previous cases reported in literature. In this regard, to the best of our knowledge, it has never been reported the association between BPDs, pachychoroidal neovasculopathy and polypoidal choroidal vasculopathy, as highlighted in our patient. Although there is still no consensus on the classification of pachychoroidal disease, these imaging findings led us to make a diagnosis of pachychoroidal neovasculopathy (PNV) in which it is typical the presence of shallow and patchy RPE detachments due to the presence of the underlying CNVs [14]. In this regard, OCTA images represent a useful diagnostic tool in detecting type 1 neovascularization growing under the RPE. Thus, we deem that OCTA scans should always be performed in patients with PDs, in order to diagnose as early as possible, the presence of CNVs. As already previously stated by other authors, pachychoroidal disease, particularly PNV, should be considered as a spectrum of RPE and choroidal abnormalities which might progress and develop, as in our case, towards other more typical form of disease such as polypoidal choroidal vasculopathy. As a matter of fact, previous authors stated that PCV might be considered as a long-standing and chronic PNV [15].
In conclusion, multimodal imaging (FAF, SS-OCT, SS-OCTA, FA, ICGA) have been shown to be highly informative in understanding the natural history of a case of BPDs which turned out to be also considered as a long-standing and chronic form of PNV, progressed to PCV.
Citation: Desideri LF, Cirafici P, Traverso CE, Nicolo M (2020) Multimodal Imaging in a Long-standing Case of Pattern Dystrophy. J Clin Exp Ophthalmol. 11:864.
Received: 27-Oct-2020 Accepted: 10-Nov-2020 Published: 17-Nov-2020
Copyright: © 2020 Desideri LF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.