Journal of Bone Research
Open Access
ISSN: 2572-4916
ISSN: 2572-4916
Review Article - (2014) Volume 2, Issue 2
Background: The standard view of blood coagulation is based on a mechanism whereby cascade interactions of clotting factors generate thrombin, which converts soluble fibrinogen into an insoluble clot.
Objective: Review the modalities by which soluble fibrinogen transforms into an insoluble matrix, the basis of blood coagulation.
New concept: An alternate process is operative that can transform fibrinogen, based on reactions with free
radicals. Such could be generated by the release of ascorbate by activated platelets. Ions of multivalent metals, such as Cu+2 or Fe+2 bound to fibrinogen, react with the ascorbate (a reductant in a Fenton reaction) to generate H2O2 and reactive oxygen species. Alternately, γ-irradiation which generates H2O2 could generate such species. Supportive evidence and references are cited.
Conclusion: An expanded blood clotting schema is presented that incorporates the classic (via thrombin) as well as alternate (free radical) pathways by which fibrinogen can be converted into an insoluble clot. This new schema is discussed within the context of γ-irradiation or dietary ascorbate as instigants of free-radical induced clotting events, of particular relevance to airplane pilots, divers, submariners, astronauts and patients not responding to classic anticoagulation (heparin, Coumadin) therapy.
Keywords: Mechanisms; Thrombin; Vitamin C; Free
Keywords: Mechanisms; Thrombin; Vitamin C; Free radicals; Neofib; Thrombosis
Cβ, preCγ and CαE: Synthetic Peptide Analogues (19-21 AA) of the β, γ and αE Chain C-termini of Fibrinogen; FA: Fatty Acids; D-D: Binding Contact between the D-domains of 2 Different Fibrin Monomers; Fib340: Fibrinogen with MW 340 kDa; Fib420: Fibrinogen with Extended α Chain MW 420 kDa; [Fib]min: Minimal Fibrinogen Concentration required for Coagulation (Phase Change) to occur; FPA: Fibrinopeptide A released (Cleaved) from Fibrinogen by Thrombin; FPB: Fibrinopeptide B released (Cleaved) from Fibrinogen by Thrombin; Haptides?: Fibrino-Peptides Homologous to the Carboxy-Terminal Sequences of the β-, γ-, and αE Chains (i.e. Peptides Cβ, preCγ and CαE respectively), which can elicit Haptotactic (Attachment) responses from Cells of Mesenchymal Origin; SEM: Scanning Electron Microscopy; TEM: Transmission Electron Microscopy
The standard view of blood coagulation is based on the idea that a "cascade" of enzymatic events, the "intrinsic" and "extrinsic" pathways of coagulation both generating thrombin, which converts fibrinogen into an insoluble fibrin clot [1-7].
Consequently, much of the biomedical focus relating to blood coagulation has been on the clotting factor thrombin (clotting factor IIa), resulting from the "trypsinic" cleavage of prothrombin (clotting factor II) into thrombin (clotting factor IIa). For example, citrate is added to donated blood units to chelate Ca(II) , which prevents the activation of carboxylated pro-factors (clotting factors II,VII, IX, X) into enzymatically functioning proteins (factors IIa, VIIa, IXa, Xa respectively). For medical purposes, strategies for preventing thrombin formation include dosing with anti-thrombotic drugs, based on the idea of preventing the biosynthesis of carboxylated enzyme precursors of thrombin (with coumadins) or inhibiting thrombin activity (such as with heparin). A simplified schema of the 'classic' cascade is presented in Figure 1.
Structure and composition
Fibrinogen is the plasma protein responsible for blood clot formation. Normal fibrinogen (Fib 340) is a complex of 2 each of 3 chains (α, β and γ ), with a MW 340 kDa. A variant of fibrinogen, with a longer α chain and greater MW (known as fibrinogen αE in Fib420 ; MW 420 kDa), constitutes about 1% of the total fibrinogen in adult humans. Thus, the three normal fibrinogen chains are composed of 610, 483, 411 amino acids and the aE chains are 866 aa (the numbering based on the Gene-bank database, accessible at ncbi.nlm.nih.gov).
Two molecular representation of Fib340 and Fib420 are shown in Figure 2
The α chains in Fib340 fold back over the molecule, hovering near the central E-domain. The much longer αE chain of Fib420 also folded back, but presents globular features over the E-domain.
Fibrinogen has a strong affinity for divalent metal cations, notably Ca (II) and Zn (II), which at physiologic levels (around 2 mM and 10 uM respectively) can each modulate the rate of fibrinogen coagulation (discussed further below) and the physical properties of the resultant fibrin clot, such as viscoelasticity or breaking strength [9-13].
New graphic icons of fibrinogen are presented below (Figure 3) as pseudo "Newman projections" used by organic chemists to describe isomeric sugars. The E domain of fibrinogen is represented by a filled circle with 2 sets of A & B "knobs" emanating from the E-domain surface. In fibrinogen, these "knobs" are covered by the terminating epitopes which are released as fibrinopeptides (FPA and FPB), following activation by thrombin. The D-domain is drawn as 2 circles, a hydrophobic region (yellow) within the other, representing the 2 receptor "holes", into which the "knobs" from the E-domain of other monomers become inserted (D:E contacts).
The Haptide epitopes terminating the β and γ chains are functional in terms of assembling fibrin monomers and complexing them with fibrinogen. Synthetic peptide analogues were shown to strongly self-aggregate and to penetrate the membranes of mesenchymal cells [14]. Thus, fibrinogen, which expresses 4 such epitopes, may attach directly to the membranes of mesenchymal cells, without the intervention of integrin receptors. This may underlie the cell attraction properties of matrices fabricated from native or denatured fibrinogen [15,16] used to harvest or culture mesenchymal cells.
Thrombin activation
The addition of thrombin to a solution of fibrinogen results in sudden phase change determined to be clot time (CT) (Figure 4) which has been monitored by turbidity, viscoelasticity, confocal and electron microscopy (SEM, TEM).
Figure 4: Conversion of clear fibrinogen solution into a turbid, viscoelastic clot, by mixing with thrombin (A). (B) Clot time (CT) is measured as the time to phase change (coagulation) after thrombin addition. C and D are images from scanning electron microscope (SEM) and transmission electron microscope (TEM) examination of fibrin (Marx unpublished)
From a mechanistic perspective, following the addition of thrombin to fibrinogen, intermediate multimer assemblies of monomers (protofibrils) with degrees of polymerization (DP) ranging between 2 to over 40 fibrin(ogen) units have been observed by electron microscopy. Such soluble protofibrils were shown to become coagulated by divalent cations, such as Ca (II) and Zn (II) [12,13].
Kinetics
An unusual feature of thrombin (or reptilase a snake venom that acts similar to thrombin) induced fibrin clot formation that it is biphasic as seen below (Figure 5).
Figure 5: Typical experimental log-log CT-Fib curves at a fixed level of enzyme (thrombin or reptilase at 1 U/ml), showing the biphasic dependency of CT on fibrinogen concentration. [Fib] min=0.2 μM was determined from the concentration below which coagulation could not be detected (i.e. CT >600 sec) [17]
Attempts to describe such biphasic fibrin coagulation kinetics by classical polymeric kinetics had been unsuccessful in predicting the experimental clot times (CT) over a large range of initial fibrinogen levels of clinical whole blood or plasma samples or with more concentrated fibrin sealants (>50 mg/ml). Thus, there was general agreement about the overall mechanism of gelation, but a credible simulation of fibrin coagulation rates (clotting time, CT) eluded exposition.
A simple reaction equation might be as in reaction 1:
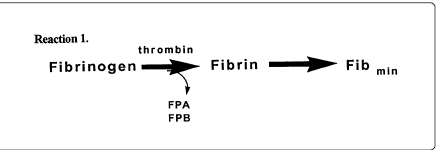
In the interest of simulating the time to phase change (CT), one would like to know the minimal concentration of fibrinogen ([Fib]min) required to be able to detect a fibrin clot.
Thus, the above reaction equation 1 generates straight (negative slope) lines in terms of dependency of CT on fibrinogen concentration ([Fib]) and thus do not reflect the whole story implied by the biphasic curve.
A more appropriate equation for the transformation of soluble fibrinogen into the insoluble, thrombin-activated, fibrin clot (Equation 2) can be described [9] as follows:
Equation 2:
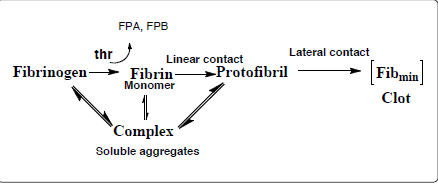
The minimal concentration of fibrinogen required for thrombin-induced clot formation (measured as clotting time) was experimentally determined to be [Fib] min=0.2 ± 0.05 µM [17]. The Haptide epitopes at the termini of the β and γ chains attract each other and help assemble soluble fibrin-fibrinogen complexes. The Haptide (chain termini) binding interactions help establish both linear and lateral D:D contacts required for the formation of the minimal 3-D clot characterized by "clotting time". The flexibility of the hydrated fibrinogen molecule ensures that once a "knob-hole" bond is established, minor conformational contortions (flexing) permit Haptide epitope contacts to more firmly dock one fibrin monomer to another, resulting in the formation of linear protofibrils, followed by lateral types of bonding, all leading to the formation of a fibrin clot with characteristic banding and branching ultrastructure (Figure 4C). A simulation of this type of reaction scheme along with inclusion of the parameter [Fib] min generated a biphasic curve that mimicked the experimental data [17].
In whole blood thrombin is generated on the surface of platelets. The physicality of the clot is further affected by the presence of red blood cells (RBC) which form rouleaux (like stacks of poker chips) [18,19]. These add mechanical strength to the clot, much like gravel and stones add to the mechanical strength of concrete (Figure 6).
In whole blood, platelets act as cellular instigators of the coagulation. They bind fibrinogen via integrins (GP IIb IIIa) and their lipidic surface provides the basis on which the various clotting factors activate each other.
The above Figure 1 encapsulates the standard view of blood coagulation – based on the cascade interactions of pro-enzymes and co-factors which generate thrombin, which in turn converts soluble fibrinogen into an insoluble fibrin clot.
New coagulation paradigm
However a redundant system is available to perform a near-equivalent transformation of fibrinogen, based on a redox reaction with vitamin C, as follows:
It is generally overlooked that platelets store high levels of vitamin C (ascorbate) in vesicles or granules (Table 1), which they release upon activation (as by exposure to a foreign surface). One might ask: What does this have to do with blood coagulation?
| Trace components in blood (51-56) | ||
|---|---|---|
| Ascorbate | Copper | |
| Blood plasma | 0.085 mM | to 1.6 uM |
| Platelets | 2-490 mM | not reported* |
Table 1: Summarizes the distribution of ascorbate and copper in blood plasma and platelets.
Scurvy, a nutritional disorder due to a deficiency of vitamin C, was the scourge of sailors who did not eat uncooked fruits and vegetables on long voyages (before 1800 when limes and citrus fruits were discovered to arrest this problem , hence the term "limey" for English sailors). The earliest symptoms of scurvy are bleeding of the gums and hematomas. As most people in our society eat some fresh fruits (juices) and vegetables regularly, scurvy or ascorbate deficiency is not an endemic problem and its connection to blood coagulation has been noted, but generally overlooked as a key pathway [20-22].
Neofib
The Fenton reaction [23-29] is one wherein a multivalent metal such as Cu or Fe reacts with an reductant such as vitamin C, to generate H2O2 and reactive oxygen species, as described in the extensive literature only slightly cited here.
Ascorbate reaction with fibrinogen
A series of experiments was designed to explore the possibility that a Fenton-type reactions could instigate the coagulation of fibrinogen, as follows. Pure fibrinogen solution was mixed with a trace amount of Ca+2 or Cu+2 or Zn+2 (10 uM) in a tris/saline buffer so as not to chelate the metals. Addition of small quantities of vitamin C (ascorbate) resulted in the immediate turbidity only with Cu+2 reflecting a Fenton-reaction resulting in protein phase change (precipitation). Further examination of this phenomenon revealed that the fibrinogen had been substantially modified by chain breaks, peptide release and the formation of new carbonyl groups both in the precipitate and in the soluble peptides in the supernatant that could be identified by SDS-PAGE analysis and reactions with dinitrophenylhydrazine (DNPH) (Figure 7) [30-34].
Figure 7: A Reaction of fibrinogen with Cu+2 and Vitamin C (Ascorbate) in a series of test tubes, obeserved as protein precipitation and turbidity (generating insoluble neofib). B. Peptide release during typical reaction of fibrinogen with ascorbate. Peptide FPA-epitope release was determined by immuno-HPLC, keto carbonyl (C=O) detection by DNPH (Marx, unpublished). C. Electrophoresis SDS-PAGE (reduced) of human fibrinogen and albumin which had been exposed to such Fenton-type reaction cycles with 100 uM ascorbate, up to 10X for albumin, which did not precipitate but became degraded as indicated by the smearing of the parent molecule into smaller fragments (Marx, unpublished)
A consideration of these results suggests a scheme whereby fibrinogen complexed with a trace amount of Cu+2 can be modified by a Fenton reaction instigated by ascorbate, as shown in Figure 8. The figure also indicates that other sources of H2O2 could also instigate a Fenton reaction to generate OH. Radicals which rapidly modify proteins as described above. For example it is known that fibrinogen is quite sensitive to redox modifications as well as to irradiation, which induce protein chain breaks, precipitation and new C=O formation [15,16,30-50].
Figure 8: Schematic representation of Fenton-type reaction with either Cu+2 or Fe +3, wherein vit C generates H2O2 (or derived from irradiation) resulting in reactive oxygen species (i.e. OH radicals), which react vigorously with the fibrinogen, converting it to insoluble neofib and releasing peptides (Marx unpublished)
The Schema (Figure 8) shows how the vitamin C reduces the C(II) to Cu(I), which can react itself becoming the Asc' radical that converts oxygen and water into H2O2, the basis for Cu(I) generating the OH'. Alternately, H2O2 formed by irradiation could in turn generate OH'. As far as fibrinogen is concerned, either mode could render it insoluble.
From the point of view of hemostasis, any phase change (such as gelation or precipitation) which occurs in blood can be termed "coagulation".
"Coagulation" is defined here as a phase change, wherein free-flowing soluble protein (such as fibrinogen) can become induced to "gel or precipitate".
Thus, it is important to take into consideration that fibrinogen can be induced to precipitate or clot by multiple modes. The characterization of free radical reactions with fibrinogen, and their role in physiologic blood coagulation, is far from complete. The Fenton reaction is a well-established reaction with thousands of references thereto, only a few of which are cited here. Interestingly, this reaction also in used to instigate in the coagulation of sewage [51]. The realization that ascorbate accumulation by platelets which permits the generation of free radicals after platelet activation [2-7] provides a biologic context for considering the reaction of vitamin C with fibrinogen as relevant to blood coagulation, as summarized below.
The values indicate that extremely high levels of ascorbate are accumulated in platelets, which are released upon activation to further coagulation. *While analytic data is not available for platelet copper stores (due to inappropriate fixation of samples with gluteraldehyde) [55], results showing high Ca and phosphate levels suggest that Cu is also stored in the dense granules, secreted upon activation.
A new blood clotting schema (Figure 9) is presented, that incorporates both the classic cascade pathways (via thrombin) as well as an alternate (via free radical) pathways by which soluble fibrinogen can be converted into an insoluble matrix or clot.
Figure 9: New blood coagulation schema via multiple mechanisms: A: Classic clotting cascades (extrinsic/intrinsic) which generate thrombin which converts soluble fibrinogen into an insoluble fibrin clot. B: Fenton reaction with vitamin C (Ascorbate) and Cu+2, which generates reactive oxygen species, such as OH. Radicals, transforming the fibrinogen into insoluble neofib (unpublished TEM images, Marx circa 1991). ¥ irradiation of a Cu+2-fibrinogen solution, under conditions that form H2O2 could result in reactive oxygen species, transforming soluble fibrinogen into insoluble precipitate (neofib) (Marx, unpublished)
Lessons to be learned
To conclude, blood coagulation (i.e. the conversion of soluble fibrinogen into an insoluble protein,) is not the sole purvey of thrombin acting on fibrinogen, but can also be instigated by reactive oxygen species. For an organism dependent of the free flow of blood through blood vessels, either pathway can result in a blood clot (embolism) with potential life-threatening consequences.
The consequences of the expanded mechanism of coagulation are suggested as follows:
1. For patients subject to run-away coagulopathies, it may be that the standard treatments with heparin or Coumadin (warfarin) may not be effective. Rather, one could consider metal-chelate therapy as well as dietary reduction of vitamin C (no fruit juices).
2. Personel exposed to high oxygen concentrations (deep sea divers or submariners) could also consider preventive metal chelate treatments before exposure. This would minimize susceptibility to Fenton-induced coagulation resulting in irreversible "bends-like "symptoms (no fruit juices).
3. Personel exposed to high radiation environments (airplane pilots, astronauts) could also consider metal chelate pre-treatment prior to exposure (no fruit juices).