
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Short Communication - (2022)Volume 12, Issue 1
We describe a patient presenting multiple organ dysfunction syndromes complicating a refractory cardiogenic shock caused by massive methadone intoxication. Neither ECG changes nor echocardiography abnormalities suggesting the diagnosis of Takotsubo syndrome could be observed at the admission. The QT interval was also normal. An Extracorporeal Life Support (ECLS) was rapidly implanted in bridge to decision. The myocardial failure was fully reversible after 10 days. This case is the first to report multiple organ dysfunction syndromes complicating refractory cardiogenic shock related to methadone poisoning requiring ECLS. It emphasizes that a diagnosis must be imperatively suspected in patients with history of drug abuse and for whom methadone is rendered available even in absence of typical ECG and/or echocardiography changes.
Methadone poisoning; Refractory cardiogenic shock; Hybrid ECLS; Multiorgan dysfunction syndrome
Methadone is a synthetic opioid used for treating opioid addiction. It is possible that cardiotoxicity has been widely reported and includes principally QT interval prolongation leading potentially torsade de pointes [1]. Hemodynamic instability, acute heart failure or more rarely cardiogenic shock have also been described following methadone overdose [2,3]. The underlying mechanism remains unclear [1]. An excessive catecholamine release responsible for Takutsubo syndrome stress cardiomyopathy has been proposed to explain acute heart failure following poisoning as well as withdrawal methadone [2,4]. Here, we describe a patient with multiple organ dysfunction syndromes complicating refractory cardiogenic shock caused by massive methadone intoxication without typical ECG changes and/or echocardiography abnormalities and in whom an Extracorporeal Life Support (ECLS) was required.
A 39-year-old man with history of drug addiction had been weaned for 7 years and had lived in Thailand for 2 years. He was found by his family on the floor in a deep coma. Upon arrival of the mobile Intensive Care Unit, the patient’s vital signs were as follows: Blood pressure 75/45 mmHg, respiratory rate 6 min-1, heart rate 76 bpm, pulse oximetry 44%, temperature 32.6°C, capillary glycaemia 0.5 g.L-1, and pinpoint pupils. The medical team found a large number of empty methadone blisters, corresponding to a total dose of 1820 mg. The patient received one dose of naloxone 10 mg; his Glasgow Coma Scale (GCS) score then increased from 3 to 13/15 with acute agitation. After tracheal intubation, the patient exhibited profound reventilation collapse, and a norepinephrine infusion of 0.8 µg.kg-1.min-1 was given in association with epinephrine to restore blood pressure during the transport to the Intensive Care Unit (ICU). Upon arrival in the ICU, his vital signs were blood pressure 137/106 mmHg, heart rate 116 bpm, and temperature 33.5°C, and SpO2 oxygen saturation 69% under 100% FiO2. Laboratory investigations showed pH 7.13, PaCO2 61 mmHg, PaO2 63 mmHg with SaO2 96%, lactates 6.0 mmol.L-1, glycaemia 0.88 g.L-1, urea 7.3 mmol.L-1, creatinine 148 µmol.L-1, procalcitonin 1.95 µg.L-1, bilirubin 45 µmol.L-1, ALAT 69 UI.L-1 (6-55), lipasaemia 226 UI.L-1 (8-78), creatine phosphokinase 1295 UI.L-1 (30-200), myoglobin 6585 µg.L-1 (<155), ethanolaemia <0.1 g.L-1, leucocytes 27.4 G.L-1, haemoglobin 15 g.L-1, platelet count 320 G.L-1. The chest X-ray revealed acute pulmonary oedema (Figure 1). Transthoracic echocardiography showed Left Ventricle (LV) failure: Dilated LV, visual LV ejection fraction 25%-30%, LV outflow tract velocity time integral 9 cm at 120 bpm, absence of mitral or aortic valvular disease, and right ventricular function preserved with absence of pulmonary hypertension. An initial electrocardiogram showed sinus tachycardia at 120 bpm with PR 160 ms, QRS 96 ms, QTc 369 ms, and upright and peaked P waves in leads II and III favouring a “P-pulmonale” wave (Figure 2).
Figure 1: The chest X-ray revealed acute pulmonary oedema.
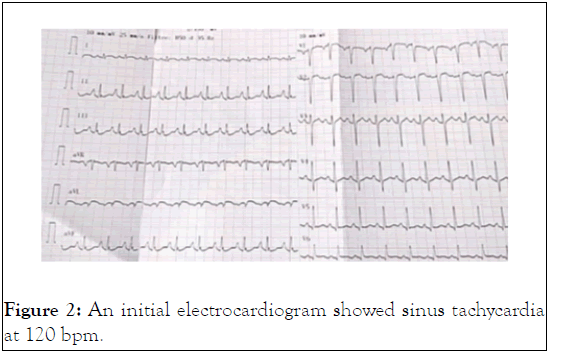 Figure 2: An initial electrocardiogram showed sinus tachycardia at 120 bpm.
Figure 2: An initial electrocardiogram showed sinus tachycardia at 120 bpm.
In the next few hours, the patient exhibited severe cardiogenic shock while the dose of epinephrine was increased to 2.1 µg.kg-1.min-1. His blood gases showed deterioration as follows: pH 7.19, PaCO2 34 mmHg, PaO2 60 mmHg with FiO2 100%, and lactates 8.3 mmol.L-1. His vital signs were blood pressure 94/74 mmHg, heart rate 123 bpm, and temperature 34.6°C, accompanied by anuria, cyanosis, and diffuse mottled skin. Thus, an ECLS device was implanted to restore end-organ perfusion with a rotaflow centrifugal pump® (Maquet cardiopulmonary AG, Hirrlingen, Germany). A 19-Fr cannula (Maquet getinge, Gothenburg, Sweden) was introduced in the left common femoral artery, a 21-Fr venous cannula (Maquet getinge, Gothenburg, Sweden) was introduced in the left femoral vein, and distal reperfusion for the patient’s leg was introduced in the superficial femoral artery. Because the patient exhibited severe hypoxemia, a 17-Fr cannula (Maquet getinge, Gothenburg, Sweden) was introduced in the patient’s left internal jugular vein to ensure precardiac oxygenation and limit the onset of Arlequin syndrome [5]. The ECLS implantation was guided by the use of transoesophageal echography. The ECLS total blood flow was initiated at 5 L/min while the jugular line was partially clamped to limit its flow to 1 L.min-1. The first blood gas sample under venous-arterial-venous ECLS (VAV-ECLS) showed pH 7.26, PaCO2 31.5 mmHg, PaO2 338 mmHg, and lactates 12.8 mmol.L-1. Empirical antibiotic treatment with meropenem and amikacin was introduced because of the risk of emergence of multidrug-resistant bacteria from Thailand.
On ECLS day 1, epinephrine and norepinephrine infusions could be reduced to 0.1 µg.kg-1.min-1 and 0.3 µg.kg-1.min-1, respectively. An intravenous infusion of dobutamine was then initiated at 5 µg.kg-1.min-1. The visual Left Ventricular (LV) ejection fraction was estimated at 15% with global and profound hypokinesia. An antero-lateral thrombus was strongly suspected. An intra-aortic balloon pump was introduced for optimisation of left ventricle unloading. It was chosen in place of Impella® because of the intraventricular thrombus. On ECLS day 2, a 24 hours levosimendan continuous infusion at a rate of 0.2 µg.kg-1.min-1 without loading dose. After this infusion, the dobutamine was reduced to 2.5 µg.kg-1.min-1 and the epinephrine infusion was discontinued, while norepinephrine was maintained to ensure mean arterial pressure near 60-65 mmHg. A significant resolution of pulmonary edema could be rapidly observed on ECLS day 2 (Figure 1). The flow of VAV- ECLS by the jugular vein cannula was set for PaO2 Í? 90 mmHg and SpO2 95%-98%. Ventilation was set at 6 ml.kg-1 of Ideal Body Weight with PEEP 8-10 cm H2O. Pulmonary oedema resolved rapidly with ECLS. The patient exhibited some organ failures including acute kidney injury (anuria, peak of creatinine 192 µmol.L-1) and hepatic cytolysis (ASAT 4306 UI.L-1 and ALAT 1162 UI.L-1) without bilirubin elevation. The prothrombin time failed at 45%, with 22% factor V. Troponin-I (ARCHITECT height sensitive troponin-I, normal <30 ng.L-1) was measured on the 1st day at 80 ng.L-1; it was 834 ng.L-1 4 hours later. The peak was obtained at 5746 ng.L-1 on the 2nd day; the level decreased immediately in subsequent days and normalised on the 5th day. The serum creatinine peak was reached on the 1st day (192 µmol.L-1); the level then gradually decreased and was normal after the 3rd day without dialysis.In response to the patient’s rapid improvement, the jugular cannula and the intra-aortic balloon pump were removed on ECLS day 3 (thus converting to venous-arterial ECLS). The venous-arterial ECLS was totally removed on the 5th day without reintroduction of inotrope or pressor amines. Liver function was normal on the ICU day 4. The patient did not exhibit any neurological injury. He was extubated on the ICU day 6 with normal arterial blood gases; oxygen was removed on the following day. Finally, the patient was discharged from the ICU and transferred to the psychiatric unit on the 10th day without any infusion of drugs or oxygen supplementation. His ECG on the 10th day was normal: 59 bpm, PR 168 ms, QRS 90 ms, QTc 391 ms, normal axis, and normal ST segment and T waves.
Radiological examination
Echocardiography gradually improved, with normalisation on day 10. Neither signs of regional wall motion anomalies nor signs of genetic hypertrophic or obstructive cardiomyopathy could be observed. Coronary angiography demonstrated normal arteries. The cranial CT-scan was normal, and CT-scan angiography did not show pulmonary embolism.
Serology tests
Bronchoalveolar lavage was negative for SARS-CoV-2, influenza virus A and B and metapneumovirus. Serology findings were negative for Brucella, Chlamydia psittaci, Chlamydia trachomatis, Coxiella, Rickettsia, syphilis, zika, toxoplasma, HIV, HBV, HCV, HHV6, and EBV. PCR analysis of blood samples revealed negative results for adenovirus, CMV, B19, zika, chikungunya, EBV, and HSV1 and HAS2. Blood cultures were also negative. Dengue serology was positive with IgM antibody at 23.191 and IgG antibody at 23.402 (ELISA, normal value <11). These serology findings were compatible with a recent infection in the past 3 months, but two blood PCR values 1 day apart were negative. Auto-immune myocarditis test results (Anti-cytoplasmic neutrophil antibody and antinuclear antibody) were negative.
Toxicological analysis
Blood samples were subjected to toxicological screening using ultraperformance liquid chromatography–high-resolution timeof- flight mass spectroscopy. The analytical system consisted of an Acquity I class system (Waters, Milford, MA, USA) coupled to a Xevo XS G2 QTOF analyser (Waters). Data were analysed with an exact mass database from Waters for >1500 toxicologically relevant drugs and metabolites. The following drugs were identified: acetaminophen, methadone and its metabolite EDDP, diazepam and its metabolite nordiazepam, etomidate, and laudanosine. Quantifications were performed for acetaminophen (immunoanalysis), methadone, and EDDP (liquid chromatography–tandem mass spectrometry). The respective plasma concentrations of methadone and EDDP upon arrival in the ICU were 1723 ng.mL-1 and 167 ng.mL-1. These high levels decreased progressively during the following 10 days. The plasma concentration of acetaminophen after the 4th hour was normal (7.1 μg.mL-1 for a therapeutic concentration between 10 and 20 μg.mL-1). One month later, a segmental toxicological hair analysis was performed (liquid chromatography-tandem mass spectrometry). Hair analysis was negative in all segments for illicit drugs and substitution therapy, corresponding to an absence of drug consumption before admission (0 to 6 cm of hair).
Previous case reports described cardiogenic shock following methadone overdose, which was treated with inotropic drugs [2,3]. The use of ECLS has been previously reported to treat opioid-induced pulmonary edema [6]. The present case is the first to report multiple organ failure syndrome complicating refractory cardiogenic shock treated by hybrid (veno-arteriovenous) ECLS. The severity of cardiogenic shock of our patient may be easily explained by the high plasma level of methadone (i.e. 1723 ng.mL-1 at the ICU admission) which has been described as lethal. Although the underlying mechanism of methadone cardiotoxicity remains unclear, QT prolongation and torsade de pointes have been described [1]. In despite of ingestion of large dose of methadone, our patient did not experience QT prolongation. As previously reported, cardiac function was fully restored after 10 days. The presumed cause of rapidly reversible cardiogenic shock was Takotsubo adrenergic cardiomyopathy which was previously described in patients with methadone poisoning as well withdrawal [2,4]. It should be pointed out that our patient did not exhibit the typical echocardiographic pattern (e.g., apical ballooning; mid ventricular, basal, or focal wall motion abnormalities; or regional wall motion anomalies) or ECG changes. Despite the absence of cardiac magnetic resonance imaging findings, the profound but rapidly reversible cardiogenic shock could be related to Takotsubo syndrome [7].
Methadone has very good bioavailability (80%) with a long halflife (7-65 hours) and is eliminated by the kidney (15%-40% during the first 24 hours). Our patient developed acute renal failure, which may have led to enhanced methadone accumulation and toxicity. The drug is also metabolized by cytochrome P450 3A4 and CYP2D6 [8]. However, our patient had not taken any substances that could interact with these cytochromes.
Regarding toxicological analysis results, the patient’s hair did not show evidence of substance use between 3 weeks and several months prior to the incident, which allowed us to rule out the long-term use of other drugs. Plasma analysis confirmed the absence of toxic substances other than those administered to the patient during treatment.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Beurton A, Imbault J, Concalves R, Repusseau B, Ouattar A (2022) Multiple Organ Dysfunction Syndrome Complicating Refractory Cardiogenic Shock caused by Methadone Overdose and Treated by Extracorporeal Life Support (ECLS). J Clin Toxicol. 12:502.
Received: 04-Jan-2022, Manuscript No. JCT-20-7065; Editor assigned: 07-Jan-2022, Pre QC No. JCT-22-7065 (PQ); Reviewed: 18-Jan-2022, QC No. JCT-20-7065; Revised: 24-Jan-2022, Manuscript No. JCT-22-7065 (R); Published: 31-Jan-2022 , DOI: 10.35248/2161-0495-22.12.502
Copyright: © 2022 Beurton A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.