
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Review - (2021)Volume 12, Issue 1
COVID-19; Methylene blue; Zinc; Photoinactivation; SARS-CoV-2; Lysosomotropism
The SARS-CoV-2 pandemic requires solutions to limit the yet unrestrained effects of the viral infection. Repurposing drugs to address COVID-19 can provide an alternative to limiting the pathogenesis of such zoonotic spillover [1-8]. As the efforts to control the contagion in the context of a relatively distant perspective from global herd immunity, a legacy from the struggled minds of three Nobel laureates may potentially contribute to limiting the viral associated pathogenesis and spread.
The German physician-scientist Paul Ehlrich has exploited several antimalarials in his endeavor of finding a proper “magic bullet” [9]. One of them, Hydroxychloroquine has already showed potential benefits in addressing enveloped viral pathogens such as SARS-CoV- 2 [10]. However the pleioptropic effects of a yet unexploited antimalarial in the form of a phenothiazinum derivative photosensitizer could potentially bring additional advantages to the current approaches in combating the pandemic [11-13].
The Belgian physician-scientist Christian de Duve, while deciphering the structural and functional organization of cells, has elegantly described the property of certain cationic drugs to become trapped in acidic organelles such as lysosomes following the process of protonation which leads to a decrease in the activity of low pH endo-lysosomal hydrolases. Since lysosomal hydrolases are necessary for the uncoating of the envelope and fusion step of many enveloped viruses, lysosomotropism has long been regarded as a potential antiviral approach [14]. Enveloped viruses exploit the endosomal pathway for the fusion with their hosts from within endo-lysosomes and coronaviruses are not an exception from this mechanism [15] hence lysosomotropism can contribute to limiting this process [16,17].
The Danish-Faroese physician-scientist Niles Ryberg Finsen, in his endeavor of exploiting the biological effects of light radiation in the form of phototherapy [18] has revealed the ability of certain agents to enhance the biological effects of light, as photosensitizers. This opened a new avenue for the medical field and since, the photooxidizing effect of light in combination with photosensitizers has been used in many medical procedures, including the inactivation of viruses in blood products for transfusions [19].
MB, a phenothiazine derivative antimalarial [20] and photosenzityzer [13] meets many of the principles evaluated in the works of these three Nobel laureates which make it a potential agent with multiple uses in addressing the pathogenesis of enveloped viruses. These include its ability of accumulating in lysosomeslysosomotropism, carrying metals across lipid membranes-metal ionophore and inactivating viruses under the action of visible lightphoto inactivation, with potential benefits in the management of resilient enveloped viruses including the new member of the beta coronavirus family, the SARS-CoV-2.
Lysosomotropism
Lysosomotropism is a property of weak bases such as amines and cations, which ensures their ability of accumulating in acidic organelles by protonation [21]. Cationic antimalarials impede parasitic and viral pathogens by accumulating in the lysosomes of the pathogens or their hosts and reducing the activity of lowpH dependent hydrolases leading to the impairment of envelope uncoating or the vacuolization of the organelle [14,22]. The principle of the process was elegantly described by the discoverer of lysosomes, the 1974 Nobel Prize laureate Christian de Duve [23]. The antimalarial and antiviral effects of cationic drugs such as chloroquine and phenothiazine derivatives such as MB24 are mostly attributed to lysosomotropism [20,23]. MB is capable of lysosomotropism accumulating preferentially in endo-lysosomes due to its cationic properties [25-27]. While the ability of binding to nucleic acids and accumulating in acidic organelles is mostly exploited in vital cellular staining, [28] the lysosomotropic property of MB can be exploited in inhibiting viral uncoating in acidic cellular organelles. This mechanism was indicated by de Duve for most lysosomotropic agents [23] and was soon demonstrated experimentally by other authors [14]. Both the endosomal and non-endosomal pathways become exploited for cell entry by coronaviruses depending on the tissue type, [15,29] so the inhibition of endo-lysosomal acidification by lysosomotropic agents such as MB could potentially limit the pathogenesis of SARS-CoV-2 from narrowing its tissue tropism [30].
Metal transport across lipid bilayer membranes
Lipid bilayer membranes represent physical barriers for cells, cellular organelles and the envelope of viruses. A variety of carriers (metal ionophores) can passively transport metal ions across these membranes. Common antimalarials are Zn2+ ionophores and quinoline derivatives like chloroquine and phenothiazine derivatives like MB are known for possessing this ability [31,32]. Zn2+ is a metal with inhibitory effects on the elongation of the RNA dependent RNA polymerase, a constituent of RNA viruses which is foreign to the human body. Its inhibitory effect was demonstrated for SARS-CoV in vitro [33] and suggests that MB and Zn2+ formulations could potentially overcome physical barriers and exert antiviral properties against coronaviruses by inhibiting the viral replicase in many of its states, either directly on free virions, on virions exploiting the endo-lysosomes or, in the replication compartments enclosed by lipid bilayer membranes (Figure 1) [34].
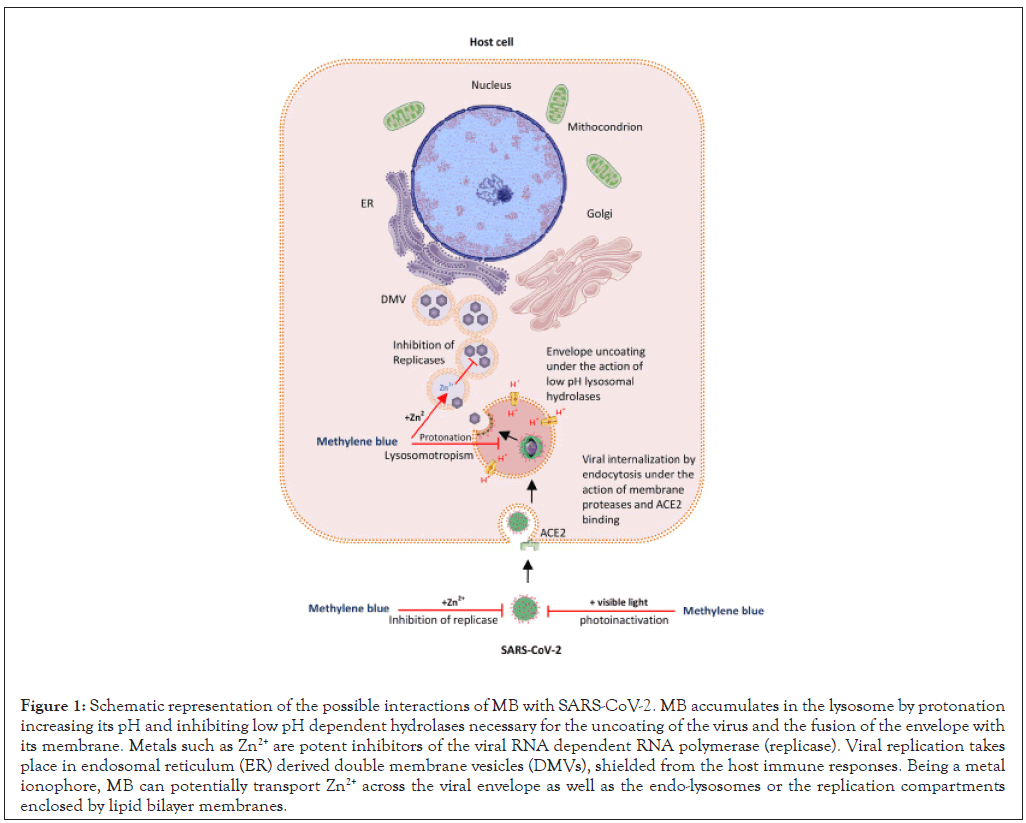
Figure 1: Schematic representation of the possible interactions of MB with SARS-CoV-2. MB accumulates in the lysosome by protonation increasing its pH and inhibiting low pH dependent hydrolases necessary for the uncoating of the virus and the fusion of the envelope with its membrane. Metals such as Zn2+ are potent inhibitors of the viral RNA dependent RNA polymerase (replicase). Viral replication takes place in endosomal reticulum (ER) derived double membrane vesicles (DMVs), shielded from the host immune responses. Being a metal ionophore, MB can potentially transport Zn2+ across the viral envelope as well as the endo-lysosomes or the replication compartments enclosed by lipid bilayer membranes.
Viral photoinactivation
Light radiation exerts biological effects on live systems and photosensitizers can amplify such effects. This principle has been widely described and exploited by the 1908 Nobel prize laureate Niels Finsen, leading to the emergence of a new field for the medical science in the form of phototherapy [35]. MB is a recognized photosensitizer which induces a photo-oxydizing effect of nucleic acids under visible light [36]. This effect has long been established for the photoinactivation of virions in blood products designed for transfusions, being efficient against every tested virus [12,19,27] including the more recently identified SARS-CoV-2 [11]. It’s oxidation by visible light results in reactive oxygen species (ROS), mostly singlet oxygen, leading to depurination, guanosine oxidation and strand cross-linking, with consequent viral neutralization. Although MB is being used in photodynamic therapy for several types of cancers due to spontaneously accumulating in lysosomes and moving to the nucleus after the application light, [27] the viral photoinactivation procedure is indicated for the free virions in the plasma to avoid cell lysis. However, the photoinactivation procedure for viruses was reported to be safe even for whole blood [37]. Combining hemofiltration or dialysis with MB based photoinactivation could represent the perspective of safely purging the plasma of patients with high viral loads. The use of an aerosolized form of MB solution under visible light could target the viral load in the ambient air neutralizing or attenuating virions in enclosed spaces and such formulations could further exploit the effect of added heat or alcohol which may contribute to altering the integrity of the envelopes lipid bilayer, while still remaining a safe method for decontamination of ambient air. This could even result in active immunization if the attenuated viruses do manage to enter their hosts. Besides, photoinactivated or attenuated viruses resulting from using MB and visible light could be used in the protocols of producing vaccines against various enveloped viral pathogens including SARS-CoV-2. By degrading the nucleic acids of viral structural proteins and genome, MB solution decontamination could provide an advantage over the current procedures using alcohol, chlorine or UV light, since currently, residual integer viral RNA on fomites could test positive on RTPCR analysis [38] even if they remain non-pathogenic [39]. The usage of a combined chemical (MB, alcohol) and physical (heat, visible light) approach for decontamination becomes justified in the context of dealing with a virus resilient to physical aggressions [40].
Oxidizing stress
Oxidizing stress can lead to the accumulation of reactive oxygen species in cells. This can result from viral aggressions, oxidizing drugs and other physical and chemical aggressions. An important enzyme of the cellular antioxidative system which is responsible for the reduction of ROS is glucose-6-phosphate dehydrogenase (G6PD). Its deficiency is associated with the consequences of the accumulation of ROS, ultimately leading to cell lysis. Hemolytic anemia is one of the most important complications which occur in G6PD deficient patients, being triggered by infections, oxidizing drugs and fava beans, favism (G6PD deficiency) being the most common enzymopathy, with a prevalence of approximately 5% worldwide [41]. The implications of G6PD deficiency in COVID-19 are far from being completely understood but a link has been suggested between the two entities [42]. Oxidative stress induced methemoglobinemia can occur as a consequence of oxidized hemoglobin to methemoglobin and is associated with clinical signs of tissue hypooxygenation such as cyanosis in the skin and oral mucosa, fatigue, headache at doses between 15%-30%, dyspnea and tachypnea, confusion, syncope at doses between 30%-50%, and cardiac palpitations due to arrhythmias and chest pain due to ischemia, confusion, cognitive impairment and convulsions at doses between 50%-70%. This can further be aggravated by oxidizing drugs such as antimalarials, Aspirin, Nitrofurantoin, Nonsteroidal anti-inflammatory drugs (NSAIDs), Quinidine, Quinine and Sulfa drugs, leading to oxidative hemolytic anemia [43,44], and levels of more than 70% methemoglobinemia can be lethal [45].
The most widely used antidote for methemoglobinemia in emergency settings is MB in small antioxidant doses of 1-2 mg/ kg, up to 50 mg total. This is done with the reserve that methylene blue itself becomes an oxidant drug at doses higher than 7 mg/kg IV and can cause methemoglobinemia in susceptible (e.g. G6PDH deficient) patients at doses higher than 5 mg/Kg [46,47].
MB has the potential of targeting multiple pathways of the viral infectious cycle of enveloped viruses which resides in its abilities of lysosomotropism, Zn2+ transport across lipid bilayer membranes, viral neutralization through photoinactivation and reduction of ROS. Different formulations of MB alone or in combinations with Zn2+ for prophylaxis, visible light, heat, and alcohol for decontamination and extracorporeal circulation and dialysis for treatment could represent potential uses of MB in addressing the pathogenesis of multiple enveloped viral pathogens including SARS-CoV-2. Being a safe agent for human administration at therapeutic doses, MB becomes a suitable agent to be adapted to both therapeutic and prophylactic settings in order to limit viral replication and reduce the severity of infections with enveloped viruses. Although its use may not completely prevent the infection or treat the disease, exploiting the potential of MB to impair the functioning of enveloped viruses may contribute to the limitation of their pathogenesis to milder forms of the disease. In the light of these attributes, MB could represent a valuable tool to be be exploited in the management of COVID-19 and other zoonotic outbreaks and the arguments for its usage reside in the principles described by the previous works of the Nobel laureates of the 20th century-Paul Ehlrich, Niels Finsen and Christian de Duve, prompting for clinical trials to evaluate its pleiotropic antiviral effects.
The authors declare they have no actual or potential competing financial interests.
There is no applicable funding from public or private sources for the writing of this manuscript.
All authors contributed to the articles conception. The first draft of the manuscript was written by C.C.A. who had the idea for the article and performed the literature analysis while CG, FSN, MSN and IBN critically revised the work. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Citation: Andrei CC, Laurentiu CG, Fazel NS, Mohammad NS, Ioana BN (2021) Multiple Potential Targets of Methylene Blue against Enveloped Viruses: Lessons from Three Nobel Laureates. J Clin Cell Immunol. 12:611.
Received: 22-Jan-2021 Accepted: 05-Feb-2021 Published: 12-Feb-2021 , DOI: 10.35248/2155-9899.21.12.611
Copyright: © 2021 Andrei CC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.