Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Case Report - (2021)Volume 9, Issue 10
Myeloid sarcoma (MS) is a rare form of extramedullary myeloblasts infiltration which can affect various organs resulting in multiple complications with high mortality. We reported two cases of MS as extramedullary relapse in patients with Acute Myeloid Leukemia (AML). The first case was 45-years old female with AML M2 who developed symptoms of central nervous system, hepatobiliary, and vision impairment after three months of CR. Imaging studies revealed leptomeningeal, optic nerve, hepatobiliary, lymph nodes, and bone infiltration suggestive for MS. The second case was 52 years old female with AML M3 presented lower abdominal bulge and vaginal bleeding resulting from uterine cervical MS one year after CR. Awareness of MS as extramedullary AML relapse is very crucial for timely diagnosis and treatment despite its very poor prognosis.
Myeloid sarcoma; Acute myeloid leukemia; Relapse
AML: Acute Myeloid Leukemia; MS: Myeloid Sarcoma; CR: Complete Response; CT: Computed Tomography
Myeloid sarcoma (MS), chloroma, granulocytic sarcoma, myeloblastoma, or extramedullary myeloid tumors, is a pathologic diagnosis for extramedullary blast cell proliferation from one or more myeloid germ line which disrupt the normal architecture of infiltrated tissues [1]. The incidence reached up to 2.5-8% and more common in certain subtypes of AML including M5a, M5b, M4, and M2 [2]. It can affect any system of the body, commonly involved lymph nodes, skin, soft tissue, testicles, bone, peritoneum and gastrointestinal tract, and can occur in several anatomical locations [3,4]. MS can present as isolated MS or part of AML relapse and diagnosis is confirmed by immunohistochemistry of the biopsy sample of affected organ. Despite being highly infrequent, the prognosis of MS is poor especially with relapse of AML [5].
Case 1
A 45-year old female diagnosed with AML M2 in August 2019 Bone Marrow Aspiration (BMA) showed in Figure 1.
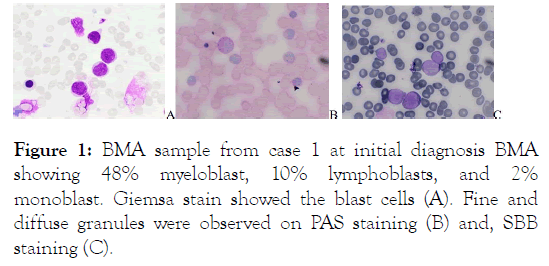
Figure 1: BMA sample from case 1 at initial diagnosis BMA showing 48% myeloblast, 10% lymphoblasts, and 2% monoblast. Giemsa stain showed the blast cells (A). Fine and diffuse granules were observed on PAS staining (B) and, SBB staining (C).
The patient had undergone induction chemotherapy with regiment 3+7 in November 2019 and achieved Complete Remission (CR) (Figure 2).
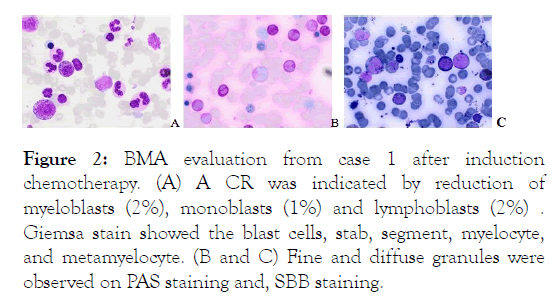
Figure 2: BMA evaluation from case 1 after induction chemotherapy. (A) A CR was indicated by reduction of myeloblasts (2%), monoblasts (1%) and lymphoblasts (2%) . Giemsa stain showed the blast cells, stab, segment, myelocyte, and metamyelocyte. (B and C) Fine and diffuse granules were observed on PAS staining and, SBB staining.
Three months later, she complained headache, slurred speech, and blurry vision. Peripheral blood revealed a white blood count of 40 x 103/μL with 47% blasts, and blood chemistry showed marked increase in liver transaminases and bilirubin. Abdominal CT scan revealed intrahepatic cholestasis, metastatic nodule in the liver and abdominal lymph nodes, and lytic lesion of the femur head (Figure 3).
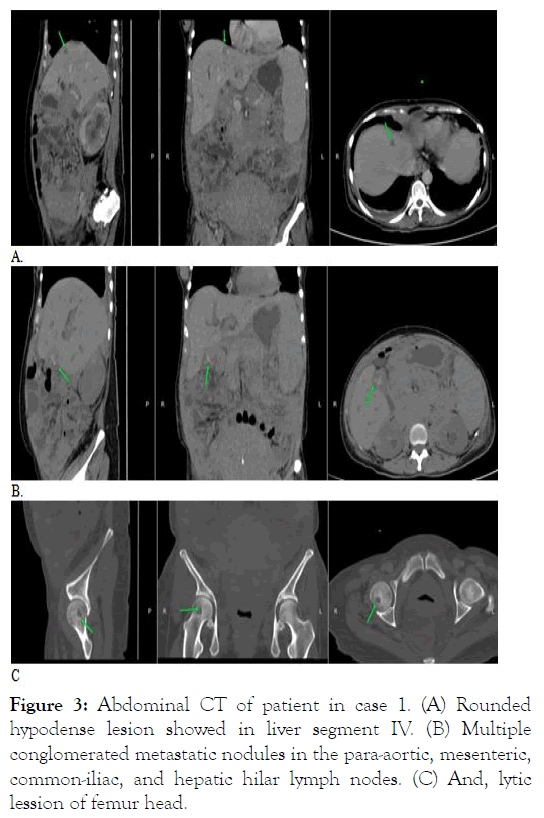
Figure 3: Abdominal CT of patient in case 1. (A) Rounded hypodense lesion showed in liver segment IV. (B) Multiple conglomerated metastatic nodules in the para-aortic, mesenteric, common-iliac, and hepatic hilar lymph nodes. (C) And, lytic lession of femur head.
Contrast brain CT suggestive of leptomeningeal metastasis and funduscopy showed infiltrative optic neuropathy in both eyes (Figures 4 and 5).
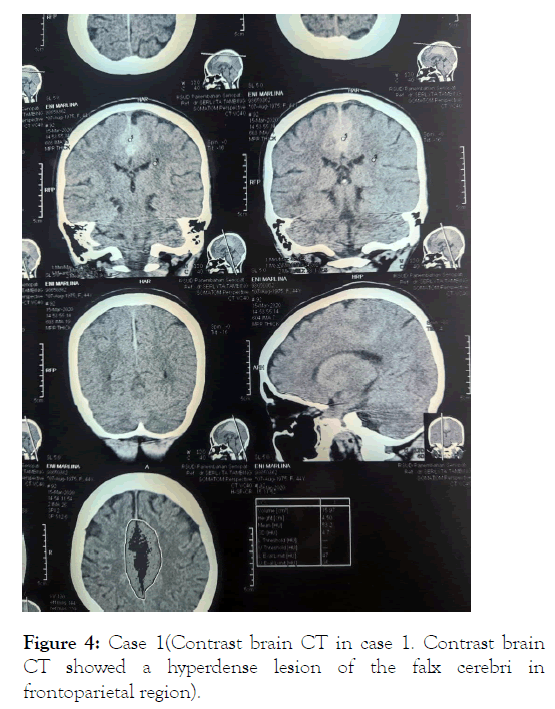
Figure 4: Case 1(Contrast brain CT in case 1. Contrast brain CT showed a hyperdense lesion of the falx cerebri in frontoparietal region).
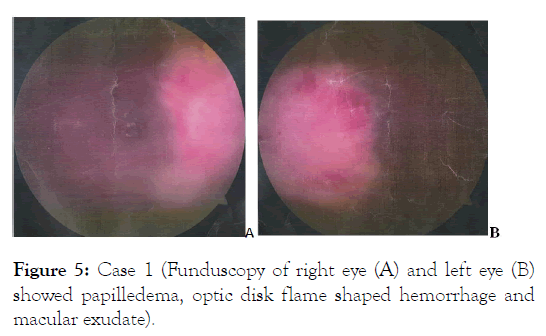
Figure 5: Case 1 (Funduscopy of right eye (A) and left eye (B) showed papilledema, optic disk flame shaped hemorrhage and macular exudate).
The patient was diagnosed with AML relapsed with MS in the ocular, leptomeningeal, hepatobiliary, lymph nodes, and bone, with amaurosis fugax due to infiltrative optic neuropathy. Second induction chemotherapy was planned, however the patient deteriorated very quickly due to neurological complication, kidney failure, worsening pneumonia and urinary tract infection. She passed away on the twelfth day of hospitalization due to uncontrolled sepsis with multi-organ failure.
Case 2
A 52-year old female was diagnosed with AML M3/ Acute Promyelocyte Leukemia in November 2017 BMA showed in Figure 6.
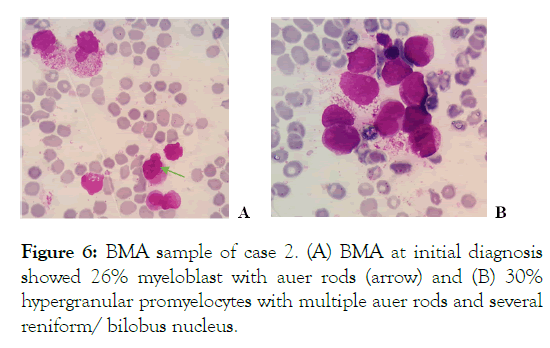
Figure 6: BMA sample of case 2. (A) BMA at initial diagnosis showed 26% myeloblast with auer rods (arrow) and (B) 30% hypergranular promyelocytes with multiple auer rods and several reniform/ bilobus nucleus.
She achieved CR after induction chemotherapy with 3+7 regimen, followed by consolidation therapy with ATRA (Figure 7).
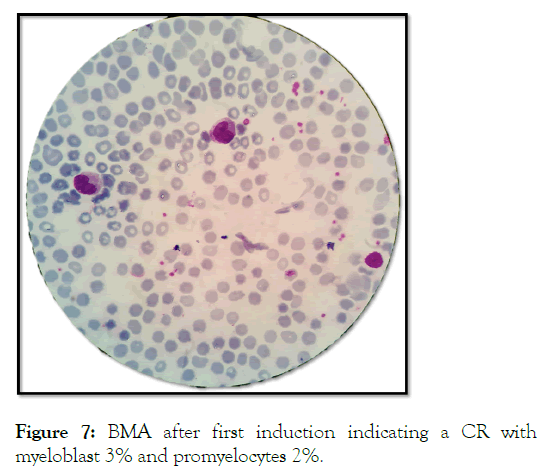
Figure 7: BMA after first induction indicating a CR with myeloblast 3% and promyelocytes 2%.
After 2 months, ATRA was discontinued due to hearing disturbance and skin peeling problem. One year later (April 2019), she presented with painless bulge in the lower abdomen and vaginal bleeding. Ultrasound showed echoic mass in the uterine cervix suggestive for malignancy (Figure 8).
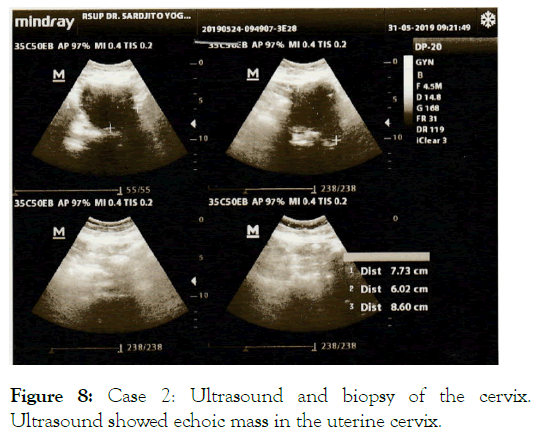
Figure 8: Case 2: Ultrasound and biopsy of the cervix. Ultrasound showed echoic mass in the uterine cervix.
Uterine cervix biopsy was performed Figure 9, and IHC staining was positive for CD117 and CD34, confirming the diagnosis of uterine cervix MS.
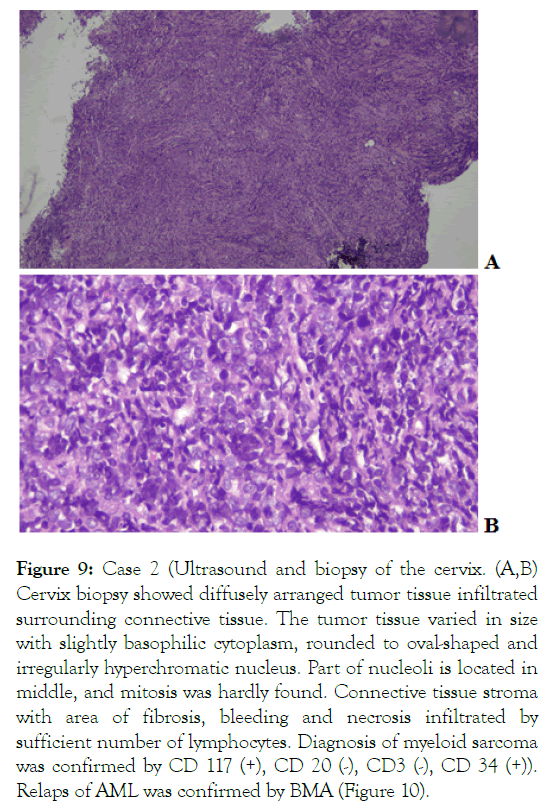
Figure 9: Case 2 (Ultrasound and biopsy of the cervix. (A,B) Cervix biopsy showed diffusely arranged tumor tissue infiltrated surrounding connective tissue. The tumor tissue varied in size with slightly basophilic cytoplasm, rounded to oval-shaped and irregularly hyperchromatic nucleus. Part of nucleoli is located in middle, and mitosis was hardly found. Connective tissue stroma with area of fibrosis, bleeding and necrosis infiltrated by sufficient number of lymphocytes. Diagnosis of myeloid sarcoma was confirmed by CD 117 (+), CD 20 (-), CD3 (-), CD 34 (+)).
Relaps of AML was confirmed by BMA (Figure 10).
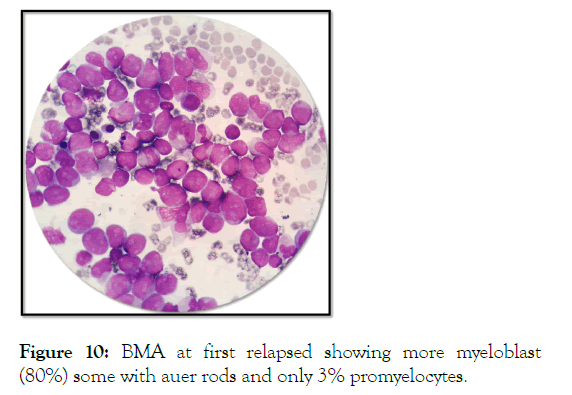
Figure 10: BMA at first relapsed showing more myeloblast (80%) some with auer rods and only 3% promyelocytes.
After second induction with 3+7 regimen followed by consolidation therapy with ATRA, she achieved CR again with shrinking of uterine cervical MS although PAP smear remains positive for tumor cells. In September 2019, she presented a recurrent vaginal bleeding, increased myeloblast in peripheral blood, and progression of uterine cervix mass (Figure 11).
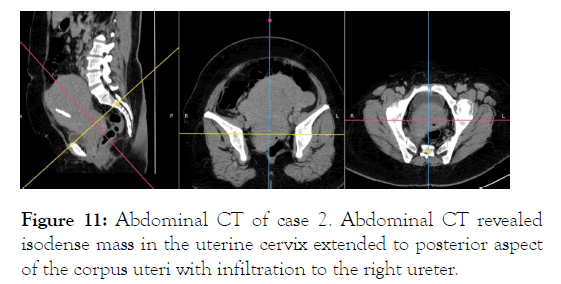
Figure 11: Abdominal CT of case 2. Abdominal CT revealed isodense mass in the uterine cervix extended to posterior aspect of the corpus uteri with infiltration to the right ureter.
The patient had second relapse and treated with cytarabine along with supportive therapy. However, in December 2019, she had worsening pneumonia and passed away due to sepsis.
According to WHO classification, MS is included in AML and related neoplasm subgrou [6]. The ESH classified MS into: (1) MS concomitant with AML, (2) extramedullary relapse of AML, including those that occur following BM transplant; (3) blast phase/ transformation from myeloproliferative neoplasm or CMML; (4) isolated MS, which occur without history of myeloid dysplasia and with normal BMA.
The pathogenesis of MS involves alteration of homing signal on leukemic blast cells which blocks its normal localization in the BM. This process resulted from interaction between different chemokine receptors, the blast cells ability to invade its surrounding tissue, and specific interaction between matrixmetalloproteinase- 9 and beta-(2)-leukocyte surface to induce leukemic cells migration [7-9]. CD56 which is often found in t(8;21) also associated with MS [10]. Translocation t(8;21) has been related with AML-M2 morphology, which is the morphological diagnosis of our first case.
Overall, the incidence of MS in CNS is only 0.4% of total cranial BM, vertebra, or orbital bones and migrate to underneath brain parenchyma due to disruption of BB [12,13]. Brain CT of our first case showed hyperdense lesion on frontoparietal region of falx cerebri. On non-contrast CT, intracranial MS commonly appear as extra-axial hyperdense mass that also suggestive for other diagnosis including meningioma, B-cell lymphoma, and intracranial metastasis. MS also express B-cell antigen (CD19, CD79a) which potentially mislead to CNS lymphoma [12].
MS of gynecologic organs is rare, commonly affected uterus and ovaries. Vaginal, parametrium, and pelvic lymph nodes involvement also have been reported [14-16]. It can accompanied by abdominal pain, ulceration, and vaginal bleeding or asymptomatic. Radiologic finding is not spesific, and cytochemistry and IHC with myeloperoxidase, lysozyme, CD117, CD34, CD43, and CD68 can be used to confirm diagnosis [15,17]. The second case experienced lower abdominal bulge accompanied with vaginal bleeding. Uterine cervix MS was supported by ultrasound, confirmed by histopathology and positive CD117 and CD34.
Hepatobiliary MS often has symptom of obstructive jaundice, acute liver failure, nausea, vomiting, and/or upper quadrant abdominal pain. Jaundice may resulted from obstruction or compression of biliary duct by MS [18,19]. Radiologic finding of hepatobiliary MS is atypical; diagnosis mainly confirmed with liver biopsy or from autopsy [20]. Our first case manifested intrahepatic cholestasis with hepatic nodules suggestive of leukemic infiltration.
Optic nerve involvement in hematologic malignancy is common due to its nature as “pharmacologic sanctuary” with a prevalence of 18% in acute leukemia and accompanied by other systemic or neurologic symptoms [21,22]. In the first case, infiltrative optic neuropathy resulted in amaurosis fugax which is a visual emergency that can result in permanent blindness. Establishing a definite diagnosis can be challenging since the clinical manifestation of blurry vision or sudden visual loss may resemble other causes of optic nerve injury [23]. Brain and orbital MRI should be performed promptly to rule out compressive lesion and to determine disease extension. Optic nerve biopsy should be considered in severe visual loss if initial examination failed to conclude diagnosis. A routine ocular examination should be performed on every patient with leukemia [24].
The Lymph Nodes (LN) are one of the most commonly involved area in MS [1]. Mesenteric LN involvement has been reported in several reports, and the most frequent CT finding were lymphadenopathy in mesenteric, retroperitoneal, para-aortic, pelvic, and inguinal area [24,25]. A comprehensive approach is essential to differentiate MS and lymphoma in extensive lymphonodal involvement due to the histologic similarity of blast cell and large cell NHL particularly in poorly differentiated MS. Diagnosis is confirmed with immunophenotyping and chromosome analysis of tissue biopsy [26]. Our first case represented MS in the LN extensively besides in other above mentioned organs.
There is no consensus treatment of MS due to its rarity and lack of RCTs. Treatment depends on tumor location, timeline of MS (before AML onset or relapse of AML), age, and performance status. Therapeutic modalities include chemotherapy, surgery, radiotherapy, allogenic haematopoietic stem cell transplantation (allo-SCT), targeted therapy, and immunotherapy [10]. Surgery is important to relieve symptom of mass effect, diagnosis confirmation, and debulking of large-sized MS before systemic therapy [3]. Radiotherapy for isolated MS is recommended when adequate response cannot be achieved from chemotherapy and rapid relieve for vital function impairment e.g. VCSS and MESCC [1,10]. In our first case, the prominent symptoms of seizures, uremic syndrome and multiple infections halted this treatment option. The recommended chemotherapy regiment is conventional AML protocol with individual risk stratification adjustment. Induction chemotherapy protocol for isolated MS is not yet established, however cytarabine consisting regiment commonly used with favourable outcome [3]. Allo-SCT showed promising result in patients with MS who were in remission after first induction chemotherapy. However, the decision to perform allo-SCT at first remission should be made
There is no consensus treatment of MS due to its rarity and lack of RCTs. Treatment depends on tumor location, timeline of MS (before AML onset or relapse of AML), age, and performance status. Therapeutic modalities include chemotherapy, surgery, radiotherapy, allogenic haematopoietic stem cell transplantation (allo-SCT), targeted therapy, and immunotherapy [10]. Surgery is important to relieve symptom of mass effect, diagnosis confirmation, and debulking of large-sized MS before systemic therapy [3]. Radiotherapy for isolated MS is recommended when adequate response cannot be achieved from chemotherapy and rapid relieve for vital function impairment e.g. VCSS and MESCC [1,10]. In our first case, the prominent symptoms of seizures, uremic syndrome and multiple infections halted this treatment option. The recommended chemotherapy regiment is conventional AML protocol with individual risk stratification adjustment. Induction chemotherapy protocol for isolated MS is not yet established, however cytarabine consisting regiment commonly used with favourable outcome [3]. Allo-SCT showed promising result in patients with MS who were in remission after first induction chemotherapy. However, the decision to perform allo-SCT at first remission should be made carefully and based on individual morbidities including risk of graft-versus-host disease [27].
Prognosis of MS is still controversial with limited data. However, MS that occurs concomitantly with AML or relapse AML considered has poor prognosis [28]. For treatment regiments, patients who received chemotherapy showed a better prognosis than those who were not [29]. The life expectancy of MS varies according to age, gender, ethnicity, and anatomical location with 24% of 5-year survival rate. The most common cause of death are relapse and infection [29-31]. The patient in our first case developed MS in multiple organs only 3 months after CR, and deteriorated very quickly, while the second case presented MS a year after first CR with single gynecological organ and survived longer than the first case.
Myeloid sarcoma can occur in patients with AML who have been in complete remission and can manifest in various organs. Awareness to recognize MS in various organs in relapsed AML is essential and this diagnosis demands further individualized treatment due to the very high mortality risk.
None
The authors declare no conflicts of interest.
Citation: Hardianti MS, Lasut RA (2021) Myeloid Sarcoma Presented as a Relapse in Acute Myeloid Leukemia: Case Reports and Review of the Literature. J Leuk. 9:276.
Received: 26-Oct-2021 Accepted: 09-Nov-2021 Published: 16-Nov-2021 , DOI: 10.35248/2329-6917.21.9.276
Copyright: © 2021 Hardianti MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License,which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.