Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2013) Volume 3, Issue 4
We have first shown that oxygen release from MbO2 at near-zero O2 concentrations (p02) only proceeds when interacting the protein with respiring mitochondria. If they are separated from MbO2 solution by a semipermeable membrane, no MbO2 deoxygenation occurs. The rates of O2 uptake by mitochondria from solution in the presence of MbO2 (V1) and MbO2 deoxygenation (V2) completely coincide for different mitochondrial preparations, the native, frozen and uncoupled by FCCP, as both V1 and V2 are determined by respiratory activity of mitochondria. However, V1 and V2 reflect different processes, because they are differently affected by the proteins, like lysozyme, competing with MbO2 for binding to mitochondria. It is found that myoglobin non-specifically interacts with phospholipid sites of the outer mitochondrial membrane, while any specific proteins or protein channels for the binding to myoglobin are lacking. The pronounced ionic strength dependence of the binding implies significant contribution of coulombic electrostatics into the formation of myoglobin–mitochondrial complex. As the total charge of myoglobin molecule does not affect the MbO2 and metMb affinity for the mitochondrial membrane, the ionic strength effect must be due to local electrostatic interactions, most probably between oppositely charged groups of phospholipids (the heads) and polar myoglobin residues in the environment of the heme cavity. The shift of the oxy- / deoxy- equilibrium toward ligand free deoxymyoglobin under anaerobic conditions in the presence of mitochondrial and artificial phospholipid membranes indicates that the myoglobin-membrane interaction results in a decreased myoglobin affinity for oxygen (and increased p50), which facilitates O2 detachment from MbO2 at physiological p02 values. Thus, in the presence of respiring mitochondria, at least two kinds of myoglobin molecules should be present in the cell. Some of them are free, with the high affinity for O2, and respectively, low p50, and the others, associated with mitochondria, with lower affinities and higher p50. For effective O2 transfer from cytoplasm, the exchange between these two kinds of MbO2 molecules must be rather fast. The Km values of MbO2 binding to mitochondria (about 104 M-1 at I = 0,15) and the lifetime of the complex (tens ns) correspond well to this demand.
<Keywords: Myoglobin; Oxygen; Electrostatistics
The most important condition for functioning of all living aerobic organisms is continuous supply of sufficient oxygen. The access of oxygen to metabolic processes in cells is mediated by the respiratory proteins, tetrameric blood hemoglobin (Hb) and monomeric muscle myoglobin (Mb). Myoglobin is expressed in red muscles in response to mitochondrial demand for oxygen, transporting it from sarcolemma to mitochondria [1-3]. Particularly high Mb concentration is observed in muscles of aquatic mammals, who spend a long time underwater for food, and also in those of highland animals, living under deficient O2, which served as the basis for the hypothesis that myoglobin is the “oxygen store” for mitochondrial cytochrome c oxidase.
Since Hill [1] and Millikan [2], myoglobin has been believed to function in accordance its oxygenation curve in solution. Under normal conditions, when O2 flow from blood is sufficient for functioning of mitochondrial electron transfer chains, myoglobin binds oxygen with high affinity, and detaches it, when the partial pressure of oxygen (pO2) falls below some crucial level (the “oxygen store” mechanism). Because of this mechanism, a high muscle performance must be maintained during muscle contractions, when a normal blood flow is disturbed. As Mb concentration in muscles of terrestrial animals is rather small (calculations show that stored O2 is sufficient for only one contraction cycle), a hypothesis was also developed that myoglobin can facilitate O2 diffusion in the cell due to reversible combination of the protein with oxygen and translational diffusion of MbO2 molecules (the myoglobinfacilitated oxygen diffusion mechanism). The physiological significance of this mechanism since Wittenberg first reported evidence for it [4-7], however, has been a matter of hard controversy. Some authors argue significant contribution of the facilitated diffusion is supplying cells with oxygen, suggesting that intracellular oxygen transport rather than O2 storage, is the main function of myoglobin, while the others deny it completely [8-12].
Both the “oxygen store” and “facilitated diffusion” mechanisms of myoglobin functioning can be effective only at high Mb concentrations and very low pO2 in the cell (less than 3 mm Hg). Respectively, the level of protein saturation by oxygen (SMbO2) observed in hard working muscles should be low. For significant contribution of the myoglobin facilitated O2 diffusion, high rates of myoglobin lateral diffusion (DMb) and large gradients of MbO2 concentration, corresponding to low intracellular SMbO2 under high muscle loads, are also needed [8,11]. But modern experimental methods estimate pO2 values in hard working heart to range from 15 to 25 mm Hg (rather than 0.5–3 mm Hg, as previously thought), and SMbO2 values defined by the NMR technique do not fall below 85%, amounting to 92% of the total pool of the protein (rather than 10-30%, as found previously) [9,10,13]. The DMb values in intact skeletal muscles appeared also to be 5-10 times lower than those used in calculations of contribution of the myoglobin facilitated O2 diffusion to supplying cells with oxygen [10]. So that, this contribution is only 1.5-4% at pO2 14].
Thus, within the cell, the conditions needed for effective functioning of myoglobin according to the “oxygen store” and “myoglobin facilitated diffusion” mechanisms are lacking, which makes both mechanisms very improbable. Note that they are formulated in the framework of homogenous thermodynamics and kinetics and assume no interaction of myoglobin with any cellular structures or metabolites. Hence, the Mb affinity for oxygen is believed constant in all model calculations of its contribution to supplying cells with oxygen [8,11,15].
Oxymyoglobin interacts with mitochondrial membrane during deoxygenation
We first showed that O2 release from MbO2 at physiological pO2 values proceeds only upon direct contact of the protein with mitochondria [16,17]. If respiring mitochondria are separated from MbO2 solution by a semipermeable membrane (mitochondria are inside a closed dialysis bag), no MbO2 deoxygenation occurs even at near-zero O2 concentration (Figure 1a). Respiratory activity of mitochondria in the dialysis bag is still preserved after several hours of the incubation at room temperature, and if they are added to the MbO2 solution, deoxyMb absorption spectrum immediately appears. When mitochondria are initially added to the MbO2 solution, deoxygenation of MbO2 is observed only after some lag period (τlag), during which the MbO2 spectrum does not change (Figure 1b). The τlag duration corresponds to the time of oxygen consumption by mitochondria from the solution, which is measured in the polarographic cell under the same conditions. Thus, the rates of O2 uptake by mitochondria in the presence of MbO2 (V1) measured both polarographically and spectrophotometrically (from τlag) are virtually identical (Table 1).
Figure 1: a) Kinetics of O2 uptake by frozen rat liver mitochondria in the polarographic cell (solid curve) and variations of MbO2 spectrum (dashed curve). Experimental conditions: mitochondrial suspension, 0.9 ml, with concentration of 30 mg/ml of mitochondrial protein is put into a closed dialysis bag placed into the sealed polarographic cell with 12.5 ml of MbO2 solution (0.07 mM). Incubation medium: Tris-HCl buffer, 10 mM; sucrose, 150 mM; KCl, 100 mM; EGTA, 0.5 mM; KH2PO4, 5 mM; succinate, 15 mM, pH 7.4.
b) Kinetics of O2 release from MbO2 in the presence of frozen rat liver mitochondria.
MbO2 concentration, 0.07 mM, 1 mg/ml of mitochondrial protein. Incubation medium and experimental conditions see in a).
| Mitochondrial sample | [MbO2] | V1, polarographic | τlag, spectrophotometric | V1 из τlag, spectrophotometric | V2, spectrophotometric | V2/V1 |
|---|---|---|---|---|---|---|
| mM | μM / min | min | μМ / min | μМ / min | ||
| Native coupled | 0.11 0.25 |
15 ± 1.5 13.5 ± 1.5 |
17 ± 2 19 ± 2 |
14.7 ± 1.5 13.2 ± 1.5 |
15.6 ± 1.5 14 ± 1.5 |
1 ± 0.05 |
| FCCP uncoupled | 0.11 0.25 |
73 ± 8 78 ± 8 |
3 ± 0.5 3.1 ± 0.5 |
83 ± 8 81 ± 8 |
77 ± 8 80.5 ± 8 |
0.98 ± 0.05 |
| Frozen | 0.11 0.25 |
50 ± 5 43 ± 5 |
5.5 ± 0.5 5.3 ± 0.5 |
45.5 ± 5 47.2 ± 5 |
46 ± 5 48 ± 5 |
1.05 ± 0.05 |
| Frozen | *0.12 | _ | 6.5 ± 0.5 | 39.5 ± 4 | 21.6 ± 2 | 0.55 |
*Carboxymethylated sperm whale CM-MbO2
Table 1: The O2 uptake by rat liver mitochondria in the presence of MbO2 (V1) according to polarographic and spectrophotometric data, and the rate of MbO2 deoxygenation in the presence of mitochondria (V2).
The rate of oxygen release from MbO2 in the presence of mitochondria (V2) measured spectrophotometrically (Figure 1b) is constant during the entire period of the oxy-deoxy transition (τtr) and does not depend on MbO2 concentration (the 0-th order in myoglobin). Indeed, no reaction rate changes are observed with increasing MbO2 concentration more than twofold (Table 1) [16,17]. Lowering the reaction order in comparison with 1st-order reaction of MbO2 deoxygenation in solution (Equation 1) is inherent for different processes with participation of membranes [18].
The polarographical rate of O2 uptake by mitochondria from solution (V0) increases in the presence of MbO2 (V1 > V0), but not of oxidized metmyoglobin (metMb) (Figure 2). The MbO2 concentration in all experiments is rather high (0.25 mM), so the concentration of bound O2 is comparable with its concentration in saturated aqueous solution. However, the electrode does not register any abrupt increase in O2 concentration in the solution due to oxygen release from MbO2 during the transition of oxy- to deoxymyoglobin. It follows that bound O2 is delivered directly to mitochondria in the course of their interaction with myoglobin. The uncoupling effect of MbO2 firstly revealed by us [16] was confirmed later for horse MbO2 and native pig heart mitochondria with glutamate and malate as respiratory substrates [19]. This effect is obviously due to MbO2 interaction with mitochondrial membrane, but its nature remains unclear.
Thus, p02 fall in muscle cells alone is not sufficient for O2 cleavage from MbO2 (the “oxygen store” mechanism does not work), but MbO2 deoxygenation should proceed with active participation of the mitochondrial membrane. The rates of MbO2 deoxygenation, V2, and of O2 uptake by mitochondria from MbO2 solution, V1, measured in the presence of the native, frozen and uncoupled by FCCP mitochondrial preparations, completely coincide, since both V1 and V2 are evidently determined by the respiring activity of mitochondria, both when it is accelerated or slow down (Table 1).
The nature of the myoglobin - mitochondria interaction. The role of electrostatics
Competitive effect of different proteins on deoxygenation rate of MbO2 in suspension of mitochondria [17].
The outer mitochondrial membrane, about 50% of which is occupied by proteins, is known to be negatively charged. To verify the presence of some myoglobin-specific proteins, protein channels and phospholipid sites in the membrane, a competitive effect of different proteins on MbO2 deoxygenation rate in the presence of rat liver mitochondria (V2) has been studied. For this purpose, we used apomyoglobin (apoMb), which is structurally homologous to the holoprotein but not binding O2, and also negatively and positively charged proteins such as monomeric lactalbumin (pI 4.4), tetrameric bovine serum albumin (pI 4.7) and egg lysozyme (pI 11) to assess the role of electrostatics in myoglobin–mitochondrial interaction. At last, V2 value of chemically modified sperm whale CM-MbO2 (pI 5.2) carboxymethylated at all surface histidines was determined. Unlike native MbO2 (pI 8.3), CM-MbO2 is negatively charged at pH 7.4. Control experiments showed that all these proteins at 0.25 mM concentration do not affect the respiratory activity (V0) of the native, frozen and FCCP-uncoupled mitochondria, i.e., in contrast to MbO2, have no uncoupling effect on their electron transfer chains [17].
Sperm whale apoMb does not inhibit the rate of MbO2 deoxygenation under standard conditions, because V2 and V1 are nearly equal (Figure 3). It suggests that the mitochondrial membrane is lacking any specific proteins or protein channels for interaction with myoglobin, which is in agreement with the fact that the available databases for stable protein–protein complexes do not contain any for myoglobin [20]. It is also unlikely that any myoglobin-specific phospholipids regions of the membrane exist, because more hydrophobic apoMb would associate with them better than the holoprotein [21].
Figure 3: Effect of different proteins (0.25 mM) on rate of O2 uptake by frozen rat liver mitochondria in the presence of MbO2 solution (0.11 mM), V1 (light columns), and the rate of MbO2 deoxygenation in the presence of mitochondria, V2 (dark columns). Incubation medium and experimental conditions see in legend to Figure 1.
The negatively charged lactalbumin and BSA also have virtually no effect on the rates of both processes, V2 and V1 (Figure 3), i.e. the proteins do not compete with MbO2 for binding to the mitochondrial membrane. In contrast, highly positive lysozyme (the protein charge is +9 at pH 7.4) very strongly inhibits the MbO2 deoxygenation, by ~ 70% at equimolar concentration of lysozyme [17] and even more at its twofold excess (Figure 3), while the rate of O2 uptake from the MbO2 solution does not change noticeably even at 4-fold excess of lysozyme [17]. The strong inhibiting effect of lysozyme on V2 (without influencing V1) is convincing evidence of its effective competition with MbO2 for binding to the mitochondrial membrane.
Thus, our findings show that O2 consumption by mitochondria from solution and MbO2 deoxygenation under physiological conditions are different processes, with the respective rates differently affected by the proteins studied. Besides, these data favor absence of any specific sites on the mitochondrial membrane for myoglobin binding. The revealed influence of the charge of myoglobin itself and other proteins on the rate of MbO2 deoxygenation indicates that myoglobin, most probably, nonspecifically interacts with negatively charged phospholipids of the outer membrane and that electrostatics plays an important role in this interaction. In spite of the low net positive charge of myoglobin (~2 at pH 7.4), electrostatic interactions close to negatively charged membrane surface might increase due to a local decrease in effective dielectric permeability and pH [22].
Determination of characteristic parameters of the myoglobinmitochondria interaction by fluorescence method [23]
As the heme group of myoglobin effectively quenches emission of various donors, the quenching of intrinsic flavin and tryptophan fluorescence of mitochondria by differently liganded myoglobins was studied. Also, the fluorescence quenching of the membrane probes embedded into the mitochondrial membrane, 1-anilinonaphthalene-8- sulfonate (1,8-ANS) and 5-[3-γ-sulphopropyl-2 (3H)-benzoksazolidin)- 2-butenilidin]-1,3-dibutyl-2-thiobarbituric acid (merocyanine 540), was studied. Both fluorescent probes are widely used in studies of natural and artificial phospholipid membranes: 1,8-ANS is shown to associate both with lipids and proteins having hydrophobic domains, while merocyanines 540 (M 540) is selective only to phospholipids [24]. The physiologically active MbO2 and inactive oxidized metMb unable to bind oxygen were used as the quenchers in the pH range 6–8 and different ionic strengths.
The heme group in myoglobin is embedded into the protein; so dynamic quenching of the emission of donors by myoglobin is not possible. Energy transfer to the heme group can occur only in a quenching complex whose lifetime exceeds that of the donor fluorescence (the static quenching). We have found that in the pH 6-8 range, the flavin and tryptophan fluorescence of mitochondrial suspension is quenched neither by MbO2 nor by metMb. The crucial distance of inductive resonance energy transfer (the Förster radius) in the flavin–myoglobin heme pair is ~ 5.0 nm [24]. Since flavin containing proteins are parts of electron transport chains located in the inner mitochondrial membrane, the absence of the flavin fluorescence quenching indicates that both MbO2 and metMb do not contact the inner mitochondrial membrane, because they (like other proteins) are unable to penetrate the outer membrane of mitochondria.
The Förster radius in the tryptophan–myoglobin heme pair is also rather large, amounting to ~ 3.7 nm [24]. The absence of the quenching of tryptophan fluorescence by means of MbO2 and metMb implies that both myoglobins do not form any stable quenching complexes with proteins of the outer mitochondrial membrane. It was shown earlier [24] that, if the fluorescent probe is located in the membrane, but some protein is not bound to it, no energy transfer from tryptophans of the protein to the probe occurs. The fluorescence of two invariant tryptophans of myoglobin itself, Trp7(A5) and Trp14(A12), located 2.15 and 1.5 nm from the heme complex, respectively, is almost completely quenched by the heme (q of tryptophan fluorescence in holomyoglobin is only 1-5% of that in water) [25,26].
On the contrary, both MbO2 and metMb effectively quench the fluorescence of 1,8-ANS and M 540 probes bound to mitochondria. The quenching of 1,8-ANS fluorescence by MbO2 is shown on Figure 4a and 4b (pH 7.4). Very similar picture is observed also in the case of the quenching of M 540 fluorescence by MbO2 under the same conditions [23]. All data on quenching the fluorescence of the 1,8-ANS and M 540 probes bound to mitochondria using MbO2 and metMb as the quenchers in the pH 6-8 range are given in Table 2. Although the quenching degree of the 1,8-ANS and M 540 fluorescence by both myoglobins is approximately the same (within experimental error), the binding constant (Km) is about 1.5 fold higher for the metMb binding to mitochondria, than that for MbO2. Note that Km values found for each MbO2 and metMb as the quencher do not depend on pH in the pH 6-8 range, because they do not differ at pH 6.4 and 7.4 (Table 2). Like 1,8-ANS with a charged sulfonic group, M 540 is not able to easily penetrate the mitochondrial membrane (at least, it does not penetrate during the experiment) [27-30]. The relatively small quenching extent of the 1,8-ANS and M 540 fluorescence, 30 ± 10%, can possibly be explained by the fact that not all bound probes are able to form efficient complexes with the quencher because of heterogeneity of their binding sites and (or) improper mutual orientation of the donor and acceptor.
Figure 4: (a) Quenching of the fluorescence of 1,8-ANS probe associated with mitochondria by oxymyoglobin. 1–8 – MbO2 concentrations are 0, 3.8, 7.5, 11.1, 14.5, 17.8, 21.0 and 24.1 μM, respectively (incubation medium without succinate: Tris-HCl buffer, 10 mM; sucrose, 250 mM; EGTA, 0.5 mM; KH2PO4, 5 mM, pH 7.4.). Excitation wavelength is 360 nm, emission spectrum maximum is 470 nm, monochromator slit widths (for excitation and emission) are 5×5 nm. (b) Plot of the 1,8-ANS corrected fluorescence intensity in the spectral maximum (Fcorr) vs. MbO2 concentration (pH 7.4). Inset: determination of the constant of MbO2 binding to mitochondria in the quenching complex.
| Ligand form Mb | рН 7.4 | рН 6.4 | ||
|---|---|---|---|---|
| Km×10–4, М–1 | Quenching degree, % | Km×10–4, М–1 | Quenching degree, % | |
| 1,8-АНС | ||||
| MbO2 | 2.3 ± 0.3 | 24 ± 3 | 2.7 ± 0.4 | 20 ± 3 |
| metMb | 3.5 ± 0.4 | 31 ± 3 | 3.8 ± 0.4 | 21 ± 3 |
| MbO2 | М 540 | |||
| 2.5 ± 0.3 | 23 ± 3 | 2.8 ± 0.3 | 17 ± 3 | |
| metMb | 3.1 ± 0.4 | 37 ± 4 | 3.2 ± 0.4 | 17 ± 3 |
Table 2: Quenching of the fluorescence of the 1,8-ANS and M 540 probes associated with mitochondria by MbO2 and metMb in the pH 6-8 range (the incubation medium without succinate).
When the 1,8-ANS and M 540 fluorescence quenching by metMb is conducted under various KCl concentrations in the 10–150 mM interval, the Km values decrease with increasing ionic strength (Table 3), indicating an important role of electrostatic interactions in formation of the myoglobin-mitochondria quenching complex. Note that the Km values determined at pH 7.4 and 6.4 for different ionic strengths are identical within experimental error, i.e. do not depend on the protein total charge that varies in the pH 6-8 range.
| Ionic strength (mM KCl) | From M 540 fluorescence quenching, Km × 10-4, (М-1) | From 1,8 ANS fluorescence quenching, Km × 10-4, (М-1) | ||
|---|---|---|---|---|
| рН 6.4 | рН 7.4 | рН 6.4 | рН 7.4 | |
| 0.01 0.05 0.1 0.15 |
3.0 ± 0.3 2.2 ± 0.2 1.3 ± 0.2 1.1 ± 0.2 |
3.1 ± 0.3 2.1 ± 0.2 1.25 ± 0.2 1.0 ± 0.2 |
3.8 ± 0.4 2.5 ± 0.3 1.75 ± 0.2 1.2 ± 0.2 |
3.5 ± 0.4 2.8 ± 0.3 1.8 ± 0.2 1.25 ± 0.2 |
Table 3: Constant of metMb binding to mitochondria at pH 6-8 and ionic strength ranging from 10 to 150 mM KCl.
The fluorescence of both probes, 1,8-ANS and lipid probe M 540, is equally well quenched by MbO2 and metMb and the quenching parameters (quenching degrees and Km values of the quencher) are very close (Table 2 and 3). This favors (i) the location of both probes in phospholipid regions of the mitochondrial membrane and (ii) MbO2 and metMb binding to these areas. The lifetime of the quenching complexes should exceed the emission lifetime (τfl) of 1,8-ANS and M 540, equal to 6-9 ns [24] and ~2 ns [31]. Figures 5a and 5b show the proposed location of 1,8-ANS and M 540 in a phospholipid bilayer membrane [24,27,28]. Since the charged sulfonic groups (pKa is –1) should remain on the surface, 1,8-ANS molecule can not sink deeply into bilayer. Instead, one of its phenyl rings should be adjacent to glycerine sites of phospholipids, while the other should be contacting water (Figure 5a). The M 540 molecule consists of unsaturated hydrocarbon chain containing two ring systems, and also carries a negative charge on the sulfonic group (Figure 5b). Therefore, M 540 is located in the intermediate area of polar heads of phospholipid bilayer with anionic sulfonic group directed to the more polar outer surface of the heads. The rest of the rodlike dye is located between the ether links of phospholipids, anchoring the two butyl groups in hydrocarbon chains [28].
The clear Km dependence on ionic strength is observed (Table 3), which implies a significant contribution of electrostatic interactions into the formation of myoglobin–mitochondrial complex. The total charge of myoglobin molecule is shown not to influence the MbO2 and metMb binding to mitochondria. Then, the ionic strength effect on myoglobin affinity for the mitochondrial membrane must be due to the local electrostatic interactions, involving oppositely charged polar groups of phospholipids (the heads) and of myoglobin residues surrounding the heme cavity.
This is supported by the fact that metMb affinity for mitochondria is 1.5 fold higher than that of MbO2 (Table 2). The difference can not be explained by the ligand-induced conformational changes in the protein structure, as they are very small for differently liganded myoglobins, mainly occurring in the distal part of the heme cavity, where the ligand binds [20]. Most obviously, the differences in the electronic and charge state of the heme complex of MbO2 and metMb play major role here, namely, between the diamagnetic neutral ferrous heme in MbO2 (the Fe ligand is O2 molecule) and the paramagnetic charged ferric heme in metMb (charge +1 and the Fe ligand is H2O molecule).
The data obtained using 1,8-ANS and M 540 fluorescent probes concerning the effect of local electrostatic interactions are in good agreement with the results obtained us previously by other methods [16,17,32]. Many investigators noticed long ago that a flat pattern of seven invariant charged residues exists on myoglobin surface near the heme. The pattern is located in the bend between C and D helices and believed to serve as an anchor for myoglobin binding to mitochondria or some other cellular structures [3,33]. Besides, calculations of dipole moments of different animal myoglobins (Sivozhelezov V.S., unpublished data) show that in all cases, the positive end of the vector of Mb dipole moment (200 D) is in the area of 88–91 residues, whereas the proximal His93(F8) associated with the Fe atom is located from the opposite side of F helix. Local electrostatic interactions upon the comlexing of myoglobin with the mitochondrial membrane could take place between polar groups around the myoglobin heme cavity, ionization state of which does not change in the pH 6-8 range, and oppositely charged groups of phospholipids (the heads), which are pronouncedly zwitterionic, in this pH range. The most likely candidates to interact with anionic groups of membrane phospholipids are the invariant arginine and lysine residues in the environment of the heme cavity (Figure 6a and 6b).
The effect of phospholipid membranes on the oxy-/deoxymyoglobin equilibrium
It has long been noted by many authors that oxygen affinity of myoglobin is 4-5 times lower in muscle cells than in solution at 37°C, although no compounds are known yet that are capable to significantly change p50 of myoglobin, like diphosphoglycerate, protons and CO2 affecting p50 of hemoglobin [3,34]. Moreover, p50 values of myoglobin in rat myocytes were experimentally demonstrated to depend on the state of mitochondria [35]. Due to very high myoglobin affinity for oxygen (p50 is 0.6–0.7 mm Hg at 25°C), some changes of the oxy- /deoxymyoglobin ratio, which are caused by the presence of phospholipid membranes in solution, can be registered only under anaerobic conditions, when O2 concentration is close to zero. In our experiments, therefore, oxygen was removed from MbO2 solution in the polarographic cell by respiring mitochondria put in the closed dialysis bag (as noted above, no MbO2 deoxygenation is observed, Figure 1a). Then, at p02 in the cell near zero, bilayer phospholipid vesicles (liposomes) maid from lecithin or nonrespiring mitochondria inhibited by antimycin A were added.
In the presence of nonrespiring mitochondria inhibited by antimycin A, a decrease in the absorbance at 542 and 581 nm, which is intrinsic for MbO2 spectrum, is observed, while the absorbance at 560 nm, in the spectral maximum of deoxymyoglobin (Mb(2)) increases (Figure 7a). The shift of the oxy- / deoxymyoglobin equilibrium toward ligand free Mb(2) increases proportionally to concentration of mitochondria in the suspension, which is clearly seen from the difference spectra (Figure 7a, curves 2** and 3**). The same effect is observed in the presence of lecithin liposomes at 50-fold molar excess of lecithin (Figure 7b). It is evident that the equilibrium O2 dissociation constant (Kdis) rises in both cases due to interaction of the protein with phospholipid membranes. This conclusion is additionally supported by our findings that under aerobic conditions, MbO2 autoxidation rate (kox) also increases in the presence of nonrespiring mitochondria and artificial bilayer membranes [36], as far as a direct correlation was experimentally shown earlier between Kdis and kox [37,38].
Figure 7: The shift of the oxy- / deoxymyoglobin equilibrium in the presence of mitochondria inhibited by antimycin A (a), and lecithin liposomes (b).
a) 1– MbO2 spectrum without mitochondria (the control), 2 and 3– in the presence of mitochondria at concentration of 1 and 2 mg/ml of mitochondrial protein, correspondently, 2* and 3* – the same with deduction of the light scattering, 2** and 3** –– the difference (2* minus 1 and 3* minus 1) spectra. Incubation medium as in Figure 4, antimycin A, 20 μM, pH 7.2, 22°C.
b) 1–MbO2 without liposomes (the control), 2– MbO2 in the presence of 50-fold molar excess of lecithin, 2*– the same with deduction of the light scattering, 2** – the difference (2* minus 1) spectrum.Incubation medium as in Figure 4, pH 7.2, 22°C.
Thus, detachment of oxygen from MbO2 at physiological p02 values occurs only during interaction of the protein with the mitochondrial membrane, which results in a decreased myoglobin affinity for the ligand due to conformational changes in the heme cavity. Hence, in the presence of respiring mitochondria, at least two kinds of myoglobin molecules should be present. Some of them are free with a high affinity for O2, and respectively, a very low p50 value, and the others are associated with mitochondria and have lower affinities and higher p50 values (Figure 8). This situation should be described by the equation 1:
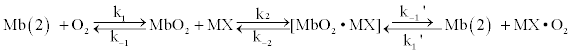 (1)
(1)
where k1 and k-1 are the O2 binding and dissociation constants of myoglobin in solution, k1’and k-1’ are those of myoglobin bound to mitochondria, and k2 and k-2 – constants of formation and decay of the myoglobin - mitochondrial complex, respectively. The difference between the k1’ and k-1’ constants and corresponding constants without superscripts is evidently due to some changes in the heme cavity conformation because of interaction of myoglobin with the mitochondrial membrane.
It should be noted that for effective O2 transfer from cytoplasm, myoglobin must not form stable complexes with the mitochondrial membrane. The exchange between the two types of MbO2 molecules, free and membrane-bound, must be rather fast, for example, to proceed at the rate of rotational diffusion of the protein, in order to change an orientation of the heme cavity relative to membrane surface. Otherwise, myoglobin could not effectively accelerate oxygen delivery to mitochondria under O2 deficit in the cell. The Km values determined for the MbO2 binding to mitochondria using 1,8-ANS and M 540 fluorescent probes (about 104 M-1 at I = 0,15), and the lifetime of the complex (tens ns) correspond well to the middle (not high) myoglobin affinity for the mitochondrial membrane. Besides, the rate of rotational diffusion of myoglobin in muscle cells is found to be only 1.5 times lower, than in dilute water solution [10,12-14], which is optimal for the effective functioning of myoglobin as the oxygen transporter.
The authors are grateful to Eugeny I. Zheltukhin (Institute of Cell Biophysics RAS) for visualization of sperm whale myoglobin spatial structures and helpful in preparing the manuscript.