Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2016) Volume 6, Issue 5
Edible coatings are generally defined as continuous matrices that can be prepared from edible materials. Italian millet contains appreciable amounts of PUFA concentrated on the top layer of the milled grains that are involved in the development of rancidity on storage. Due to their high antioxidant activity Bangalore blue grapes, green tea and tulsi were selected as edible coating material for Italian millet. Dehusked grains were coated with edible material and dried. These were then analysed for functional properties and cooking characteristics. The bulk density was in the range of 0.69-0.82. There was no significant difference between solubility, swelling power, water and oil absorption capacity. Cooking time ranged from 13.16-15.20 min. Water uptake ratio ranged from 17-24. Coatings resulted in lighter product with a slightly reduced cooking time and higher water absorption than the non-coated grains. Coatings resulted in a lighter cooked sample, slight reduction in cooking time and higher water absorption capacity. However, the cooking and functional characteristic of the grain was acceptable.
Keywords: Italian millet; Natural ingredient; Edible coatings; Functional properties; Cooking characteristics
Millets are minor cereals and form the staple food for a large segment of the population. Millet contains 9-14% protein, 70-80% carbohydrates and is rich source of dietary fibre [1]. It is a starchy food with a 25:75 amylose to amylopectin ratio and it is a fairly good source of lipids (3-6%) with about 50% of the lipids in the form of polyunsaturated fatty acids. Even though the nutritional qualities of millet have been well [2,3], its utilization for food is confined to the traditional consumers in tribal populations, mainly due to nonavailability of consumer friendly, ready-to-use or ready-to-eat products as are found for rice and wheat. One of the reasons for this is attributed to the fact that Italian millet contains appreciable amounts of PUFA concentrated on the top layer of the milled grains which are involved in the development of rancidity on storage.
Italian millet (Setariaitalica) is one of the important food crops in parts of the Indian subcontinent. It is also known as foxtail, German, Hungarian or Siberian millet. Simple processing methods like dehulling, soaking and cooking are reported to result in significant decreases in antinutrients and improved bioavailability of minerals like iron and zinc and also protein digestibility [4]. Italian millet is highly nutritious, non-glutinous, and like buckwheat and quinoa, is not an acid forming food so it is soothing and easy to digest. It is considered to be one of the least allergenic and most digestible grains available [5].
Foxtail millet is largely utilized in the form of millet rice which is obtained after the millet is dehusked and polished. Dehusking strips a significant quantity of antioxidants and fatty acids from the surface of the grain. However, during this process it exposes the fat which is principally PUFA to the atmospheric oxygen causing rapid onset of rancidity. Millet rice thus has a short shelf life.
Edible coatings are generally defined as continuous matrices that can be prepared from edible materials. They can be used as thin wraps or formed as coatings on food or between food components. In fact, edible coatings have been used for centuries to prevent moisture migration, improve food appearance and increase shelf-life. However, in recent years edible coatings have been recognized for more innovative uses. One major advantage of coatings is that several active ingredients can be incorporated into the polymer matrix and consumed with the food, thus enhancing safety or even nutritional and sensory attributes [6].
Incorporation of coated millets in food formulations have shown to improve their keeping quality. The question whether incorporation of natural antioxidants rich coatings on Italian millet brings about any change to its functional or cooking quality needs to be answered. Thus, the effect of these on functional and cooking characteristics of edible coated Italian millet was examined.
The variety of Italian millet used for the study was Prasad (SiA 326). The samples were procured from All India Coordinate Research Project on Millets, UAS, GKVK, Bangalore. Tulsi was obtained from plants maintained in Dept. of Horticulture UAS Bangalore. Other ingredients were procured from the local market in Bangalore.
Preparation and selection of coating
Most of the coating solutions/paste were prepared by slowly mixing a known amount of coating material into 75-100 ml distilled water with continuous stirring until the coating materials were suspended in the solution. The consistency of solution was such that it could be mixed by a hand. The weight of the coating substance was therefore dependent on the desirable consistency. The coating material was rubbed into the grains and mixed thoroughly till the material was uniformly distributed. This was assured by closely examining the grain visually. The procedure was standardized to ensure that there were no excess lumps of the coating material in the sample.
The weight of the coating material was recorded and respective quantity of coating material used throughout the study for treating the Italian millet grain. The quantity of the paste/solution was also recorded. This information is given in the following paragraphs for the three treatments.
Bangalore blue grapes (Vitisvinifera L.), green tea (Camellia sinensis ) and tulsi (Ocimum sanctum linn ) were selected as coating material due to their high antioxidant activity [7].
Grapes: Slurry of Bangalore blue grapes (seed, pulp and skin) which were freshly procured from the market was used. For 100 g of grains 6.25 g of grape paste was applied. The paste was rubbed into the dehusked grains manually.
Green tea: Green tea (Sencha Japanese green tea) was purchased from the market and stored in refrigerator (4ºC) till use. Green tea infusion was used for coating the grains. Green tea infusion (extract) was prepared according to procedure described by Manay and Shadakshari [8]. Hundred ml of water was heated at 85ºC in a steel container. Then 10 g of green tea was added to it. The container was covered and tea was allowed to steep for period of 3 min. The decoction was then strained and cooled. This decoction was rubbed in manually into the dehusked grains. For 100 g of grains 10 ml of green tea decoction was used.
Tulsi: Fresh leaves of tulsi were harvested from the plants maintained in Dept. of Horticulture UAS Bangalore. The leaves were dried in microwave oven for a minute. Dried leaves were powdered. 5 g of powdered tulsi was mixed with 20 ml of water. For 100 g of grains 6.25 g of tulsi paste was used. The paste was rubbed into the dehusked grains manually.
Drying of coated grains
Coated grains were dried under shade and constant stream of circulating air at room temperature (26ºC ± 2ºC, RH 40%). The grains dried within 5 h. The treated grains were stored in HDPE pouches at room temperature for storage and shelf life study and at -10ºC until analysis. A view of the samples after drying process was complete is shown in Figure 1.
Functional properties
Bulk density (g/ml): A calibrated centrifuge tube was weighed and flour samples were filled to 10 ml mark by constant tapping, until there was no further change in volume. The content was weighed and from the difference in weight, the bulk density of the sample was calculated and the results are expressed as g/ml [9].
Water and oil absorption capacity: One-gram sample was mixed with 10 ml of either distilled water or in 15 ml oil for 30 min. The contents were allowed to stand at 30ºC in a water bath for 30 min and then centrifuged at 3000-5000 rpm for 20-30 min. After centrifuging the volume of the supernatant was recorded and used for determination of water and oil absorption and the results were expressed as g/g sample [10].
Solubility and swelling power: Two hundred and fifty mg finely ground sample was thoroughly mixed with 15 ml of distilled water and heated to 65ºC (being the initial temperature at which gelatinization of starch granules begins). The content was then cooled to room temperature and centrifuged at 5000 rpm for 10 min. The soluble solids content was calculated as percentage of sample soluble in water. At the same time, this was used to calculate the swelling power, since the sample has been heated at 65°C for 30 min, the residue was weighed and the increase in weight was calculated as the swelling power of the sample at that particular temperature [11].
Cooking characteristics
The methods suggested by Bhattacharjee and Kulkarni [12] were followed to evaluate the cooking quality. Weighed sample (2 g) was placed in test tubes containing boiling water (20 ml) and heated on boiling water bath.
Cooking time: Optimum cooking time was determined by pressing few grains between two slides frequently taken from the cooking tube and noting the time when opaque core has just disappeared.
Percentage increase in weight (g): The increase in weight of the Italian millet was determined by weighing the Italian millet before and after cooking and finding the difference between the two as shown below.
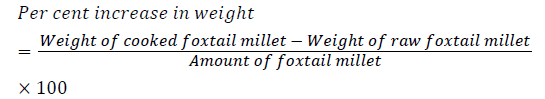
Percentage increase in volume (ml): Volume of water in a volumetric cylinder containing 100 ml of water before and after the samples cooked was measured. Increase in volume was calculated as follows:
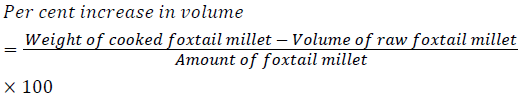
Water uptake ratio: The water uptake ratio was obtained by dividing the apparent water uptake at 80ºC by the apparent water uptake at 90ºC and the value was expressed in percentage (W2-W1) × 100.
Dispersed solids: Solids leached out into the cooking water was determined as follows. The water drained out after cooking the rice was taken in a Petri dish and dried in a hot air oven and weighed.
Weight of the Petri dish=W1
Weight of Petri dish+leached out solid after drying=W2
Therefore, total leached out solids=W2-W1
The functional properties of coated Italian millet are presented in Table 1. Bulk density of the samples ranged from 0.69-0.82 g/ml. Highest bulk density was found in uncoated Italian millet (0.82 g/ml) followed by green tea coated Italian millet (0.78 g/ml). The least bulk density was found in the tulsi coated Italian millet (0.69 g/ml). The bulk density of Italian millet variety Prasad was 0.82 g/ml which was heavier than values reported for millets. The bulk density of millet grain by Ocheme et al. [13] was 0.67 g/cm3. The values of bulk density for coated grains ranged between 0.69 g/ml and 0.78 g/ml. Thus, coating with different antioxidant rich ingredients resulted in lighter product. Significant differences were found in bulk density among the treatments (P ≤ 0.05). Lightness of the coated Italian millet can be attributed to larger air spaces between the particles when compared to uncoated Italian millet. The solubility per cent ranged from 0.74-0.76%. There was no significant difference observed between coated and uncoated samples (P ≤ 0.05). This quality has implications in product development to improve the quality traits. Water absorption capacity of coated and uncoated Italian millet ranged from 1.1-1.2 g/ml. Ocheme and Chinma [14] reported that water absorption capacity in millet flour was 1.31 g/g. Dietary fiber is known to bind water. Thus, presence of fiber in the samples contributed to its water uptake. Oil absorption capacity ranged from 0.80-1.15 g/ml. There were no significant differences in water and oil absorption capacity among different Italian millet samples (P ≤ 0.05). The coating with different ingredients did not change the characteristic of either water or oil uptake, suggesting that coated grains may be suitable for preparation of the traditional products from Italian millet.
| Treatments | Bulk density (g/ml) | Solubility (%) | Swelling power (%) | Water absorption (g/ml) | Oil absorption (g/ml) |
|---|---|---|---|---|---|
| Italian millet | 0.82 | 0.74 | 4.03 | 1.12 | 1 |
| Italian millet+Grapes | 0.75 | 0.75 | 3.99 | 1.18 | 0.92 |
| Italian millet+Green tea | 0.78 | 0.75 | 3.96 | 1.1 | 0.8 |
| Italian millet+Tulsi | 0.69 | 0.76 | 4.1 | 1.2 | 1.15 |
| F value | * | NS | NS | NS | NS |
| SEm± (0.05) | 0.0058 | 0.0128 | 0.0501 | 0.0501 | 0.151 |
| CD (0.05) | 0.0188 | - | - | - | - |
| *Significant (p=0.05); NS: Non significant | |||||
Table 1: Functional properties of Italian millet treated with different antioxidant rich ingredients.
Cooking characteristics of coated Italian millet samples were compared with uncoated de husked Italian millet are presented in Table 2. The cooking characteristics studied were cooking time, per cent increase in weight, per cent increase in volume, water uptake ratio and gelatinisation temperature. Least cooking time (13.16 min) was recorded in tulsi coated Italian millet. The type of coating influenced the cooking time in the order; green tea (15.16 min) > grapes (14.16 min) > tulsi (13.16 min). Yareshimi [15] reported that cooking time of some rice varieties ranged from 16-20 min. Thus, when compared to rice, Italian millet samples had lower cooking time. This could be attributed to its smaller size and therefore a larger surface area. The coating treatment further reduced the cooking time; this could be due to some alterations in the absorptive surface of the grain. Tulsi coated Italian millet recorded significantly higher per cent increase in weight (149%) followed by grapes (137%) and green tea (131%) coated Italian millet samples; while, it was lowest (125%) in uncoated Italian millet. Maximum per cent increase in volume (250%) was observed in tulsi coated Italian millet, followed by grapes (248%) and green tea (241%) coated Italian millet while lowest (240%) was in uncoated Italian millet. These may be due to the higher water absorption capacity of tulsi coated Italian millet during cooking as discussed in above paragraph on water absorption capacity. Pavithra [16] in her study on cooking quality of rice found that water absorption capacity was influenced by moisture content of the raw grains. This also may have contributed to the differences in values in the present study.
| Treatments | Cooking time (min) | Percent increase in weight (%) | Percent increase in volume (%) | Water uptake ratio | Dispersed solids (%) | Gelatinizationtemp (°C) |
|---|---|---|---|---|---|---|
| Italian millet | 15.2 | 125 | 240 | 17 | 2.4 | 72.1 |
| Italian millet+Grapes | 14.16 | 137.33 | 248 | 19 | 3.2 | 73 |
| Italian millet+Green tea | 15.16 | 131.25 | 240.16 | 18 | 2.6 | 72.16 |
| Italian millet+Tulsi | 13.16 | 149 | 250.33 | 24 | 3.4 | 71.06 |
| F value | * | * | * | * | * | NS |
| SEm± (0.05) | 0.15 | 0.54 | 0.67 | 0.57 | 0.05 | 0.58 |
| CD (0.05) | 0.5 | 1.78 | 2.19 | 1.88 | 0.18 | - |
| *Significant (p=0.05); NS: Non significant | ||||||
Table 2: Cooking characteristics of Italian millet treated with different antioxidant rich ingredients.
Italian millet coated with tulsi, grapes, green tea and uncoated Italian millet had water uptake ratio of 24, 19, 18 and 17 respectively. The order of dispersed solids was tulsi coated (3.4%) > grapes coated (3.2%) > green tea coated (2.6%) > uncoated Italian millet (2.4%). There were significant difference between the coated samples in water uptake ratio and dispersed solids (P ≤ 0.05). Yareshimi [15] reported that water uptake ratio and dispersed solids of some rice varieties ranged from 29.80-40.29 and 2.60-5.00% respectively. Thus, when compared to rice, Italian millet samples have lower water uptake ratio and dispersed solids. This could be attributed to amylose content. This might be also as a result of high moisture content. The coating treatment further increased the water uptake ratio and dispersed solids; this could be due to some alterations in the moisture level and absorptive surface of the grain after coating. Rice contains more moisture percentage than the Italian millet. There is relationship between amylose content and moisture to the water uptake ratio and dispersed solids [17]. The amylose content of Italian millet was reported as 17.5 per cent [18]. Gelatinisation temperature of the treated and untreated Italian millet samples ranged between 71.06ºC and 73.00ºC. Increase in starch-lipid complex formations decreases the extent of hydration in the amorphous areas, thus, resulting in an increased amount of thermal energy required for melting [15]. On cooking, the gelatinized starch tends to return from the soluble, dispersed and amorphous state to an insoluble crystalline state. It is enhanced with low gelatinization temperature and high concentration of starch [19]. The differences between Yareshmi’s values and those observed in the present study might be explained by the fact that Italian millet has a higher fat content compared to rice. The higher onset temperature for uncooked flour indicated that more energy is required to initiate starch gelatinisation from uncooked flour when compared to hydrated flour. Generally, the completion temperature increases with the protein content and melting enthalpy of starch-lipid complexes [20]. However, the values are in the same range as those reported by some other workers for millet and other cereals. Jideani and Scott [21] reported gelatinisation temperature of pearl millet (51.3-86.9) which was similar to the Italian millet. For cereal grains, the value is usually around 65-70ºC [22].
Italian millet has unique cooking as well as functional characteristics that lends itself to many conventional and modern ways of product preparation. Using edible coating in this study was a novel approach to incorporate antioxidant rich ingredients. As incorporation of these coatings did not bring about any undesirable changes in its functional and cooking characteristics it may be concluded that this approach may be acceptable for enhancing antioxidant content of Italian millet without affecting its cooking quality.