Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2022)Volume 10, Issue 5
A cross-sectional study was conducted to examine the relationship between natural radiation exposure and Intima-Media Thickness (IMT), an atherosclerosis indicator, among female residents in Karunagappally, Kerala, South India, which is known to have areas with High Natural Background Radiation (HNBR) derived mainly from thorium. Cumulative radiation doses received during childhood, adulthood, and entire life were estimated on the basis of annual indoor and outdoor radiation doses and hours spent indoors and outdoors. In 2013-2014, IMT of the carotid artery was measured with ultrasonography among 400 women aged 29-60 years living in Karunagappally. Since there were three subjects with outlying maximum IMT values, corrected IMT values excluding those outliers were calculated. For statistical analysis, raw and corrected IMT values were used. The regression analysis adjusting for age and religion showed a statistically significant association of mean and maximum IMT with radiation. The most strongly related radiation dose was with the adult dose. Its association with IMT became stronger when paediatric dose was also taken into account. When adjusted for fasting blood sugar and HbA1c, adult dose was statistically significantly related to raw mean IMT (P=0.008) and corrected mean IMT (P=0.018). Maximum IMT values were also related to adult doses but the association was not statistically significant (raw maximum IMT, P=0.061 and corrected maximum IMT, P=0.138). Among female residents in the HNBR areas in south India, mean intima-media thickness statistically significantly increased in relation to the adult dose. Further studies are necessary to evaluate the causal association of the observation.
Adult dose; Atherosclerosis; Carotid artery; High natural background radiation; Intima-media thickness; Karunagappally; Thorium
High-dose-radiation exposure damages the structures of the heart and the coronary, carotid and other large arteries [1]. Studies of Japanese atomic bomb survivors, who were exposed to atomic radiation at high dose rates, showed a dose-related excess of circulatory disease risk [2-4]. Studies of patients who underwent radiotherapy of the left breast, which involves radiation exposure at higher, yet still moderate, dose rates, demonstrated subsequent cardiovascular disease mortality risk several years post-irradiation [5].
Whether low-dose or low-dose-rate radiation exposure increases circulatory disease risk or not is the subject of debate and research [6]. One of the important epidemiological studies addressing this question is that of nuclear workers, who are usually exposed to radiation at low dose rates. The International Nuclear Workers Study (INWORKS) is by far the largest pooled analysis of nuclear worker data. Analysis of data for 308,297 nuclear workers showed a statistically-significant positive association between occupational radiation exposure and circulatory disease mortality [7]. However, mortality data are generally inadequate as the measure of the risk of non-cancer diseases because of variable case fatality. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) conducted an epidemiological evaluation of cardiovascular disease following radiation exposure and concluded as follows [8]: “Given the relatively small increase in risk associated with radiation at doses less than 1-2 Gy, it is uncertain whether epidemiological studies of mortality alone will be able to make a significant contribution to understanding the potential for and the nature of any relationship between circulatory diseases and radiation at these levels of doses.” Generally, incidence data are more reliable than mortality data with respect to disease diagnosis. However, in the case of incidence studies, it may not be easy to detect all circulatory diseases among a certain population even if the target diseases are limited to ischemic heart diseases and cerebrovascular diseases.
Important information was and still is obtained from the biennial clinical examinations conducted by the Adult Health Study (AHS) of atomic-bomb survivors in Hiroshima and Nagasaki. Using longitudinal data for about 10,000 AHS participants during the period 1958-1998, Yamada, et al. [9], examined the relationships between the incidence of non-cancer diseases and atomic-bomb radiation dose. A statistically significant dose- response relationship was found for hypertension. For myocardial infarction, a significant excess was found among survivors exposed at less than 40 years of age. Accounting for smoking and drinking did not evidently alter the results. Regarding Blood Pressure (BP), small but statistically significant effects of ionizing radiation on the longitudinal trends of both Systolic BP (SBP) and Diastolic BP (DBP) were demonstrated by a later study [10]. The AHS also revealed that serum cholesterol levels were elevated among irradiated women. Among men, only the youngest birth cohort of 1935-1945 showed a notable increase [11]. Those findings on BP’s and serum cholesterol levels might have been coincidental relationships between lifestyles and the distance from the hypocentre, rather than a causal association of radiation exposure with BP’s or serum cholesterol levels.
Intima-Media Thickness (IMT) of carotid arteries is an early marker of atherosclerosis [12]. It can predict the subsequent risk of death from myocardial infarction and stroke [13]. The AHS study examined carotid artery IMT of Hiroshima atomic bomb survivors during 2000-2012. The analysis of cross-sectional data produced an estimate of IMT per radiation dose as 0.007 mm Gy-1 (P=0.18) [14]. In a more recent AHS study, which was for the period 2010-2014, only the left internal carotid artery IMT showed a significant direct radiation effect; the IMT increase per dose was 0.09 mm Gy-1 (95% confidence interval (CI)=0.039, 0.15) [15].
The aim of the present cross-sectional study is to examine the association of cumulative natural radiation exposure dose with IMT among females in Karunagappally, Kerala, India, which is known to have areas with High Natural Background Radiation (HNBR) derived mainly from thorium.
Study population
This is a cross-sectional study done on the Karunagappally cohort by Nair, et al. [16], which includes more than 90% of Karunagappally taluk residents at the time of the baseline survey of the cohort. The taluk consists of 12 panchayats (villages). Among them, the panchayats of Alapad, Chavara, Neendakara, and Panmana have natural background radiation levels higher than those in the remaining panchayats. The median outdoor doses in those four panchayats range from 3.2 to 5.3 mGy y-1 [16,17]. As a part of the surveillance program of Natural Background Radiation Epidemiologic Study, annual medical checkups are being conducted by Cancer Care Centre, which covers the entire Karunagappally taluk [16]. In 2013-2014, the present study was conducted as a part of the annual medical checkup in the taluk.
Women aged 30-65 years who were permanent residents of the cohort area were invited for medical checkups. A total of 700 women gave written informed consent to participate in this study, and actually, 400 women underwent IMT examinations. One of them was turned out to be 29 years old, but she was also included in the present study. Women with the following medical conditions and/or medical history were excluded from IMT examinations: i) women with past or present chronic diseases of the liver and kidney, ii) women under treatment for cardiovascular diseases, valvular heart disease, atrial septal defect, ventricular septal defect, and myocardial infarction; and iii) women with heart diseases such as ischemic heart disease diagnosed on the basis of the Electrocardiogram (ECG) and auscultations at the health check-up. As a result, only 400 subjects remained for the IMT examination.
Meetings of the Institutional Scientific Review Board and Ethics Committee of Regional Cancer Centre on 12th December 2012 and 14th April 2013 approved the study, and all subjects gave written informed consent as well. The study has also received the approval from the ethics committee of Kagoshima University Graduate School of Medical and Dental Sciences. All procedures were carried out in accordance with the relevant guidelines and regulations of the above-mentioned boards.
Clinical examination
Physical examinations including the measurement of blood pressure and ECG were conducted by a medical officer from Regional Cancer Centre, Thiruvananthapuram. He was blinded to the information of the exposure dose of the subjects.
Interview: Trained registry staff team measured the height and weight of all participants and conducted interview surveys using standardized methods and with the necessary devices. The Body Mass Index (BMI) (kg m-2) was calculated by measured weight and height. Sociodemographic variables and histories of high blood pressure and diabetes were recorded in detail.
Biochemical assays: Initially, no biochemical assays were conducted. Sometime after the initiation of the study, we decided to conduct blood biochemical assays. Blood was taken in the morning after a 12 hours fasting. Initially, the following variables were examined: Total cholesterol, Low-Density Lipoprotein (LDL), High-Density Lipoprotein (HDL), and Very- Low-Density Lipoprotein (VLDL) cholesterols and triglycerides. Later in the study, we invited 200 women for the following blood biochemical assays: Fasting Blood Sugar (FBS), glycosylated haemoglobin (HbA1c), homocysteine, apolipoproteins (A1 and B) and CRP, and 164 women underwent those additional assays. The laboratory tests were performed according to the standard protocol in DDRC SLR Diagnostics Services, a laboratory in Thiruvananthapuram.
Intima–media thickness examination: The maximum IMT of the common carotid artery was determined by ultrasonography. It was done by a radiologist who was blinded to the radiation exposure status of subjects, using a high-resolution B-mode ultra- sonography device (Siemens Sonoline G30LC, India) equipped with a 10 MHz linear probe. The subjects were asked to lie down in a supine position with the neck slightly extended and head turned away from the side of examination to the respective opposite sides. Cross- and longitudinal-sections from the segments of the common carotid to the bifurcation of the internal and external carotid arteries were taken (segments were taken from 3 sites in the common carotid artery, 2 sites from bifurcation/bulb, and 3 sites from an internal carotid artery). In the present study, we used two IMT indexes, the maximum IMT and mean IMT which were obtained from a total of 16 IMT values (8 measurement points on each side of the neck).
After all the examinations were completed, those in need of medical attention were examined by a cardiologist, and those at a high risk of atherosclerosis, according to the results of health check-ups, were given lifestyle guidance, including a review of their daily diet, by a general physician.
Radiation dose estimation
An individual radiation dose of all subjects was estimated from the outdoor and indoor doses and sex and age-specific occupancy factors. The occupancy factor varies from 0.5 to 0.89 depending on sex and age, which is comparable with the value of 0.8 cited by the UNSCEAR [18].
Assuming the air kerma values for the cosmic ray component of the measured radiation level to be 0.227 mGy y-1 for indoors and 0.252 mGy y-1 for outdoors, the annual absorbed dose for each individual was calculated using the formula.
Annual dose (mGy)={(Indoor dose y -0.227) × OFindoor+ (outdoor dose y-1 (mean) of the ward or panchayat-0.252) × OFoutdoor} × CF, where OF is occupancy factor and CF is the conversion factor for air kerma to organ-specific absorbed dose presented by the International Commission on Radiological Protection (ICRP) 116 report [19,20]. Annual indoor and outdoor doses were obtained by multiplying the radiation scintillometer spot reading micro R/h with 0.0765 (=8.73 × 24 × 365.25 × 10-6) and 0.97 (TLD equivalent reading). The CF of 232Th used in the present study was 0.892 for the thyroid. The CF’s of children aged 1–14 years and infants aged less than 1 year were increased by 10% and 30%, respectively. The cosmic ray component was subtracted from the measured dose in order to estimate the radiation dose from terrestrial radiation exposure. The internal dose consisting of ingested and inhaled radionuclides was not considered for the cumulative dose estimation. The lifetime cumulative dose was lagged by 5 years. This is to allow for a possible latent period between exposure and its effects. Lifetime cumulative dose lagged by 10 and 15 years were also estimated. Adult doses were calculated by subtracting the paediatric exposure doses from their cumulative doses lagged by 5 years. For the estimation of individual paediatric exposure, the dose was accumulated from age 0 to age 14 years.
Statistical analysis
Regression coefficients, Standard Errors (SE’s), and P values were obtained from multivariable regression models. Heterogeneity test was conducted by ANCOVA.
Figure 1 shows the distributions of the maximum and mean IMT values. As shown in this figure, maximum IMT has a skewed distribution with a long upper tail. Apparently, there are three outliers. We re-calculated maximum and mean IMT values for those three individuals after the exclusion of those three outlying values. These IMT data sets will be referred to as corrected maximum and mean IMT values. Hereinafter we present two sets of results: One used the raw data set, and the other the corrected data set.
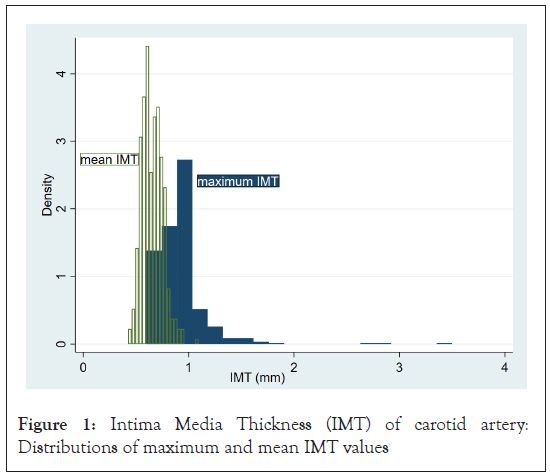
Figure 1: Intima Media Thickness (IMT) of carotid artery: Distributions of maximum and mean IMT values
Sociodemographic characteristics of study subjects are summarized in Table 1. The results of biochemical examinations are presented in Table 2.
| All | Number of subjects (%) | |
|---|---|---|
| 400 (100) | ||
| Age (years) | <40 | 71 (17.8) |
| Median=48 | 40-49 | 161 (40.3) |
| Range=29-60 | 50 | 168 (42.0) |
| Religion | Hindu | 354 (88.5) |
| Muslim | 30 (7.5) | |
| Christian | 15 (3.8) | |
| Others/unknown | 1 (0.3) | |
| Education | Illiterate | 8 (2.0) |
| Primary school | 46 (11.5) | |
| Middle school | 90 (22.5) | |
| High school | 206 (51.5) | |
| College | 47 (11.8) | |
| Unknown | 3 (0.8) | |
| Marital status | Single | 3 (0.8) |
| Married | 397 (99.3) | |
Table 1: Sociodemographic characteristics of the study subjects.
| Variable | Number of subjects | Median | Range |
|---|---|---|---|
| BMI (kg/m2) | 397 | 25 | 15-39 |
| Systolic blood pressure (mmHg) | 399 | 134 | 88-228 |
| Diastolic blood pressure (mmHg) | 399 | 74 | 45-116 |
| HDL cholesterol (mg/dL) | 322 | 50 | 20-187 |
| LDL cholesterol (mg/dL) | 322 | 143 | 45-280 |
| VLDL cholesterol (mg/dL) | 322 | 20 | 8-61 |
| Triglyceride (mg/dL) | 322 | 102 | 41-297 |
| HbA1c (mmol/mol) | 164 | 5 | 4-14 |
| Fasting blood sugar (mg/dL) | 164 | 85 | 70-262 |
| Homocysteine (mmol/L) | 164 | 18 | 9-49 |
| Apolipoprotein A1 (g/L) | 164 | 1.3 | 0.9-1.9 |
| Apolipoprotein B (g/L) | 164 | 1.1 | 0.5-2.1 |
| High-sensitivity CRP (mg/L) | 164 | 1.5 | 0.3-58 |
Note: HDL: High-Density Lipoprotein, LDL: Low-Density Lipoprotein, VLDL: Very-Low-Density Lipoprotein.
Table 2: Results of physical and clinical examinations for the study subjects.
Figure 2 shows distributions of IMT values according to age. Since IMT values increased with age, we adjusted for age in the further analyses for both data sets. Table 3 shows the age-adjusted means of IMT indexes according to religion and education. Muslim females tended to show a larger maximum IMT. For mean IMT, the association was less evident. Neither IMT index was associated with education levels.
| All | Age-adjusted mean IMT values (SE)* | ||||
|---|---|---|---|---|---|
| Mean IMT (mm) | Maximum IMT (mm) | ||||
| Raw values | Corrected values** | Raw values | Corrected values** | ||
| 0.65 (0.005) | 0.65 (0.004) | 0.91 (0.01) | 0.90 (0.01) | ||
| Religion | Hindu | 0.65 (0.005) | 0.65 (0.005) | 0.90 (0.01) | 0.89 (0.01) |
| Muslim | 0.69 (0.02) | 0.69 (0.02) | 1.02 (0.05) | 0.97 (0.03) | |
| Christian | 0.65 (0.02) | 0.65 (0.02) | 0.83 (0.07) | 0.83 (0.05) | |
| Unknown | 0.67 (0.09) | 0.67 (0.09) | 1.17 (0.26) | 1.16 (0.19) | |
| p for heterogeneity | 0.164 | 0.204 | 0.052 | 0.046 | |
| Education | Illiterate | 0.65 (0.03) | 0.65 (0.03) | 0.89 (0.09) | 0.90 (0.07) |
| Primary school | 0.67 (0.01) | 0.67 (0.01) | 0.92 (0.04) | 0.93 (0.03) | |
| Middle school | 0.66 (0.01) | 0.66 (0.01) | 0.92 (0.03) | 0.91 (0.02) | |
| High school | 0.65 (0.01) | 0.65 (0.01) | 0.91 (0.02) | 0.89 (0.01) | |
| College | 0.64 (0.01) | 0.64 (0.01) | 0.89 (0.04) | 0.88 (0.03) | |
| Unknown | 0.69 (0.05) | 0.70 (0.05) | 0.90 (0.15) | 0.92 (0.11) | |
| p for heterogeneity | 0.768 | 0.643 | 0.994 | 0.813 | |
Note : *Age adjusted mean values were obtained from regression
analyses, in which mean or maximum IMT was regressed on religion
(reference category: Hindu) or education (reference category: high
school graduates), separately, adjusting for age subtracted by 47.4 years,
which is the average age of subjects with IMT data. The regression
constants thus obtained correspond to IMT values for 47.4-year-old
Hindu women with high school education.
**Corrected IMT values were obtained after excluding IMT measurements
over 2.5 mm. P values were calculated by ANCOVA.
Table 3: Age-adjusted mean values of intima-media thickness according to religion and education.
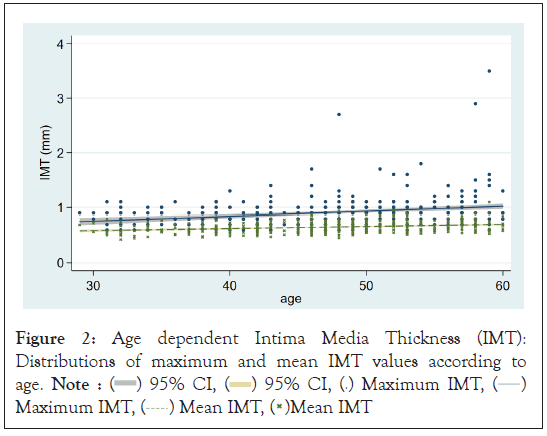
Figure 1: Age dependent Intima Media Thickness (IMT):
Distributions of maximum and mean IMT values according to
age. Note :  95% CI,
95% CI,  95% CI,
95% CI,  Maximum IMT,
Maximum IMT, 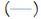 Maximum IMT,
Maximum IMT, 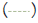 Mean IMT,
Mean IMT,  Mean IMT
Mean IMT
Table 4 summarises the results of regression analysis, in which IMT was regressed on BMI, BP’s, and serum biochemistry variables, with age adjustment.
| Variables | Regression coefficient (SE) p value |
|||
|---|---|---|---|---|
| Mean IMT (mm) | Maximum IMT (mm) | |||
| Raw values | Corrected values* | Raw values | Corrected values* | |
| BMI (kg/m2) | 0.61 (1.21) | 0.56 (1.16) | -0.15 (3.35) | -0.23 (2.41) |
| 0.614 | 0.630 | 0.964 | 0.925 | |
| Systolic blood pressure (mmHg) | 0.51 (0.23) | 0.56 (0.22) | 0.73 (0.63) | 0.99 (0.45) |
| 0.024 | 0.011 | 0.247 | 0.030 | |
| Diastolic blood pressure (mmHg) | 0.25 (0.50) | 0.31 (0.48) | 0.28 (1.38) | 0.67 (1.00) |
| 0.615 | 0.513 | 0.841 | 0.505 | |
| HDL cholesterol (mg/dL) |
-0.02 (0.30) | 0.03 (0.29) | 0.04 (0.80) | 0.38 (0.57) |
| 0.960 | 0.913 | 0.961 | 0.505 | |
| LDL cholesterol (mg/dL) |
0.21 (0.14) | 0.18 (0.14) | 0.87 (0.37) | 0.69 (0.26) |
| 0.133 | 0.187 | 0.202 | 0.009 | |
| VLDL cholesterol (mg/dL) |
0.46 (0.54) | 0.46 (0.52) | -0.72 (1.43) | -0.58 (1.03) |
| 0.396 | 0.381 | 0.616 | 0.517 | |
| Triglyceride (mg/dL) |
0.13 (0.11) | 0.13 (0.11) | -0.15 (0.30) | -0.11 (0.21) |
| 0.262 | 0.241 | 0.606 | 0.588 | |
| HbA1c (mmol/mol) |
12.43 (7.10) | 14.34 (6.67) | 4.12 (22.23) | 17.59 (14.10) |
| 0.082 | 0.033 | 0.853 | 0.214 | |
| Fasting blood sugar (mg/dL) |
0.41 (0.35) | 0.42 (0.33) | 1.43 (1.09) | 1.47 (0.69) |
| 0.247 | 0.208 | 0.192 | 0.034 | |
| Homocysteine (mmol/L) |
1.38 (1.27) | 1.33 (1.20) | 2.86 (3.94) | 2.84 (2.50) |
| 0.278 | 0.268 | 0.469 | 0.258 | |
| Apolipoprotein A1 (g/L) |
-45.72 (39.39) | -27.63 (37.28) | -112.9 (122.3) | 23.37 (78.12) |
| 0.247 | 0.460 | 0.357 | 0.765 | |
| Apolipoprotein B (g/L) |
7.57 (31.08) | 3.48 (29.34) | 86.26 (96.12) | 74.78 (61.12) |
| 0.808 | 0.906 | 0.371 | 0.223 | |
| High-sensitivity CRP (mg/L) | 0.33 (1.65) | -0.01 (1.56) | 5.66 (5.11) | 2.94 (3.26) |
| 0.843 | 0.995 | 0.269 | 0.368 | |
Note: HDL: High-Density Lipoprotein, LDL: Low-Density Lipoprotein,
VLDL: Very-Low-Density Lipoprotein.
IMT was regressed on each variable listed in the left-most column of the
table with age adjustment.
* Corrected mean and maximum values of IMT were obtained after
excluding IMT values over 2.5 mm
Table 4: Results of regression analysis for intima-media thickness.
In order to evaluate potential confounding effects on the association between the radiation dose and IMT, regression analyses were conducted with and without adjustment for potential confounding variables, and obtained regression coefficients were compared (Table 5). When raw mean IMT values were regressed on adult dose, no potential confounder affected the regression coefficients of dose by 10% or larger. When corrected mean IMT values were used in the regression analysis, FBS and HbA1c changed dose coefficients by 10% or larger. Regarding maximum IMT, in the regression of raw maximum IMT values on adult dose, FBS changed dose coefficients by 10% or larger. When corrected maximum IMT values were used, FBS and HbA1c changed the correlation coefficient by 10%.
| Variables | Adjustment* | Regression coefficients (SE) in mm Gy-1 P values |
|||
|---|---|---|---|---|---|
| Mean IMT | Maximum IMT | ||||
| Raw values | Corrected values* | Raw values | Corrected values* | ||
| All the subjects N=400 | - | 0.16 (0.04) <0.001 | 0.15 (0.04) <0.001 | 0.38 (0.12) 0.002 | 0.27 (0.09) 0.002 |
| BMI N=397 | No | 0.17 (0.04) <0.001 | 0.15 (0.04) <0.001 | 0.38 (0.12) 0.002 | 0.27 (0.09) 0.003 |
| Yes | 0.17 (0.04) <0.001 | 0.15 (0.04) <0.001 | 0.38 (0.12) 0.002 | 0.27 (0.09) 0.003 | |
| Ratio between Yes and No | 1.0 | 1.0 | 1.0 | 1.0 | |
| Systolic blood pressure N=399 | No | 0.17 (0.04) <0.001 | 0.15 (0.04) <0.001 | 0.39 (0.12) 0.002 | 0.27 (0.09) 0.002 |
| Yes | 0.17 (0.04) <0.001 | 0.16 (0.04) <0.001 | 0.39 (0.12) 0.002 | 0.28 (0.09) 0.002 | |
| Ratio between Yes and No | 1.00 | 1.07 | 1.00 | 1.04 | |
| Diastolic blood pressure N=399 | No | 0.17 (0.04) <0.001 | 0.15 (0.04) <0.001 | 0.39 (0.12) 0.002 | 0.27 (0.09) 0.002 |
| Yes | 0.17 (0.04) <0.001 | 0.15 (0.04) <0.001 | 0.39 (0.12) 0.002 | 0.27 (0.09) 0.002 | |
| Ratio between Yes and No | 1.0 | 1.0 | 1.0 | 1.0 | |
| HDL cholesterol N=322 | No | 0.18 (0.05) <0.001 | 0.17 (0.05) <0.001 |
0.43 (0.12) 0.001 | 0.32 (0.09) <0.001 |
| Yes | 0.19 (0.05) <0.001 | 0.17 (0.05) <0.001 | 0.43 (0.12) 0.001 | 0.32 (0.09) < 0.001 | |
| Ratio between Yes and No | 1.06 | 1.00 | 1.0 | 1.0 | |
| LDL cholesterol N=322 | No | 0.18 (0.05) <0.001 | 0.17 (0.05) <0.001 | 0.43 (0.12) 0.001 | 0.32 (0.09) <0.001 |
| Yes | 0.18 (0.05) <0.001 | 0.17 (0.05) <0.001 | 0.40 (0.12) 0.001 | 0.30 (0.09) <0.001 | |
| Ratio between Yes and No | 1.0 | 1.0 | 0.93 | 0.94 | |
| Triglyceride N=322 | No | 0.18 (0.05) <0.001 | 0.17 (0.05) <0.001 | 0.43 (0.12) 0.001 | 0.32 (0.09) <0.001 |
| Yes | 0.19 (0.05) <0.001 | 0.18 (0.05) <0.001 | 0.43 (0.12) 0.001 | 0.32 (0.09) <0.001 | |
| Ratio between Yes and No | 1.06 | 1.06 | 1.0 | 1.0 | |
| Fasting Blood Sugar N=164 | No | 0.11 (0.06) 0.081 | 0.10 (0.06) 0.098 |
0.28 (0.20) 0.153 | 0.16 (0.13) 0.194 |
| Yes | 0.10 (0.67) 0.128 | 0.09 (0.06) 0.16 |
0.24 (0.20) 0.242 | 0.11 (0.13) 0.389 |
|
| Ratio between Yes and No | 0.91 | 0.90 | 0.86 | 0.69 | |
| HbA1c N=164 | No | 0.11 (0.06) 0.081 | 0.10 (0.06) 0.098 | 0.28 (0.20) 0.153 | 0.16 (0.13) 0.194 |
| Yes | 0.10 (0.06) 0.142 | 0.08 (0.06) 0.191 | 0.29 (0.20) 0.156 | 0.14 (0.13) 0.272 | |
| Ratio between Yes and No | 0.91 | 0.80 | 1.04 | 0.88 | |
| Homocysteine N=164 | No | 0.11 (0.06) 0.081 | 0.10 (0.06) 0.098 | 0.28 (0.20) 0.153 | 0.16 (0.13) 0.194 |
| Yes | 0.10 (0.06) 0.109 | 0.09 (0.06) 0.133 | 0.27 (0.20) 0.182 | 0.15 (0.13) 0.252 | |
| Ratio between Yes and No | 0.91 | 0.90 | 0.96 | 0.94 | |
| Apo lipoprotein A1 N=164 | No | 0.11 (0.06) 0.081 | 0.10 (0.06) 0.098 | 0.28 (0.20) 0.153 | 0.16 (0.13) 0.194 |
| Yes | 0.11 (0.06) 0.082 | 0.10 (0.06) 0.100 | 0.28 (0.20) 0.156 | 0.16 (0.13) 0.194 | |
| Ratio between Yes and No | 1.0 | 1.0 | 1.0 | 1.0 | |
| Apo lipoprotein B N=164 | No | 0.11 (0.06) 0.081 | 0.10 (0.06) 0.098 | 0.28 (0.20) 0.153 | 0.16 (0.13) 0.194 |
| Yes | 0.11 (0.06) 0.082 | 0.10 (0.06) 0.099 | 0.28 (0.20) 0.158 | 0.16 (0.13) 0.202 | |
| Ratio between Yes and No | 1.0 | 1.0 | 1.0 | 1.0 | |
| High sensitivity CRP N=164 | No | 0.11 (0.06) 0.081 | 0.10 (0.06) 0.098 | 0.28 (0.20) 0.153 | 0.16 (0.13) 0.194 |
| Yes | 0.11 (0.06) 0.079 | 0.10 (0.06) 0.099 | 0.30 (0.20) 0.129 | 0.17 (0.13) 0.170 | |
| Ratio between Yes and No | 1.00 | 1.01 | 1.07 | 1.06 | |
Note: *In addition to each variable, age and religion were always
included as covariates. Each IMT index was regressed on the cumulative
dose lagged by 5 years.
**Corrected maximum and mean values of IMT were obtained after
excluding IMT values over 2.5 mm
Table 5: Results of regression analysis of IMT on cumulative doses with or without potential confounding variables.
Tables 6 and 7 show the results of analysis in which mean IMT was regressed on radiation doses using raw and corrected data sets, respectively. The most strongly related radiation dose was the adult dose and its association with mean IMT became stronger when paediatric dose was also taken into account. Even after adjusting for FBS and HbA1c, the adult dose was statistically significantly related to raw mean IMT values (P=0.008) and corrected mean IMT values (P=0.018).
| Regression coefficient (SE), mm Gy-1 P value |
||||
|---|---|---|---|---|
| All subjects (N=400) |
Subjects with FBS and HbA1c information (N=164) | |||
| Explanatory variable(s): Radiation dose | Model 1* | Model 1* | Model 2** | |
| Cumulative dose lagged by 5 years |
0.16 (0.04) <0.001 | 0.11 (0.06) 0.081 |
0.10 (0.07) 0.141 |
|
| Cumulative dose lagged by 10 years | 0.18 (0.05) 0.001 |
0.11 (0.07) 0.147 |
0.09 (0.08) 0.235 |
|
| Cumulative dose lagged by 15 years | 0.19 (0.06) 0.002 |
0.10 (0.09) 0.282 |
0.07 (0.09) 0.408 |
|
| Pediatric dose | 0.19 (0.12) 0.133 | -0.07 (0.18) 0.703 |
-0.04 (0.18) 0.842 |
|
| Adult dose# | 0.26 (0.06) <0.001 | 0.21 (0.08) 0.014 |
0.18 (0.09) 0.037 |
|
| Pediatric dose and Adult dose## |
Pediatric dose | -0.23 (0.15) 0.139 | -0.37 (0.20) 0.062 |
-0.34 (0.21) 0.100 |
| Adult dose | 0.33 (0.08) <0.001 | 0.29 (0.09) 0.002 |
0.28 (0.10) 0.008 |
|
Note: *IMT was regressed on each radiation dose after adjusting for the effects of age and religion, **IMT was regressed on each radiation dose after adjusting for the effects of age and religion among subjects with FBS information, #Adult doses=cumulative dose (lagged by 5 years)– pediatric dose, ##Both pediatric and adult doses were included in a regression model.
Table 6: Results of regression analyses for mean IMT (raw data) according to the period of radiation exposure.
|
Regression coefficient (SE), mm Gy-1 P value |
|||
|---|---|---|---|---|
| All subjects (N=400) |
Subjects with FBS and HbA1c information (N=164) | |||
| Explanatory variable(s): Radiation dose | Model 1* | Model 1* | Model 2** | |
| Cumulative dose lagged by 5 years | 0.15 (0.04) <0.001 |
0.10 (0.06) 0.098 |
0.08 (0.06) 0.180 |
|
| Cumulative dose lagged by 10 years | 0.16 (0.05) 0.001 |
0.10 (0.07) 0.177 |
0.08 (0.07) 0.293 |
|
| Cumulative dose lagged by 15 years | 0.17 (0.06) 0.003 |
0.08 (0.08) 0.331 |
0.06 (0.08) 0.489 |
|
| Pediatric dose | 0.18 (0.12) 0.129 |
-0.06 (0.17) 0.699 |
-0.03 (0.17) 0.874 |
|
| Adult dose# | 0.26 (0.05) <0.001 |
0.18 (0.08) 0.019 |
0.16 (0.08) 0.059 |
|
| Pediatric dose and Adult dose## |
Pediatric dose | -0.19 (0.15) 0.196 |
-0.34 (0.19) 0.072 |
-0.29 (0.20) 0.145 |
| Adult dose | 0.30 (0.08) <0.001 |
0.26 (0.09) 0.003 |
0.24 (0.10) 0.018 |
|
Note: *IMT was regressed on each radiation dose after adjusting for the effects of age and religion, **IMT was regressed on each radiation dose after adjusting for the effects of age, religion, FBS and HbA1c, #Adult doses=cumulative dose (lagged by 5 years)-pediatric dose, ##Both pediatric and adult doses were included in a regression model, $Corrected mean values of IMT after excluding IMT measurements over 2.5 mm.
Tables 8 and 9 summarize the results of regression analysis on the relationship between and radiation doses using raw and corrected data sets. Similar to mean IMT, the most strongly related radiation dose was also the adult dose; its association with maximum IMT became stronger when paediatric dose was also taken into account. When adjusted for FBS and HbA1c, which we think potential confounders, the adult dose was not statistically significantly related to raw or corrected maximum IMT values.
| Explanatory variable(s): Radiation dose | Regression coefficient (SE), mm Gy-1 P value |
|||
|---|---|---|---|---|
| All subjects (N=400) |
Subjects with FBS and HbA1c information (N=164) | |||
| Model 1* | Model 1* | Model 2** | ||
| Cumulative dose lagged by 5 years median=109 mGy; range=23-914 mGy |
0.38 (0.12) 0.002 | 0.28 (0.20) 0.153 | 0.25 (0.20) 0.223 |
|
| Cumulative dose lagged by 10 years median=96 mGy; range=19-822 mGy |
0.42 (0.14) 0.003 | 0.30 (0.23) 0.201 | 0.25 (0.24) 0.285 |
|
| Cumulative dose lagged by 15 years median=83 mGy; range=15-729 mGy |
0.46 (0.17) 0.006 | 0.30 (0.27) 0.268 | 0.25 (0.28) 0.370 |
|
| Pediatric dose median=39 mGy; range=12-413 mGy |
0.51 (0.04) 0.133 | 0.05 (0.55) 0.924 | 0.42 (0.55) 0.938 |
|
| Adult dose# median=69 mGy; range=10-800 mGy |
0.59 (0.17) 0.001 | 0.47 (0.26) 0.069 | 0.44 (0.27) 0.109 |
|
| Pediatric dose and Adult dose## |
Pediatric dose | -0.36 (0.43) 0.400 | -0.57 (0.62) 0.356 | -0.64 (0.65) 0.328 |
| Adult dose | 0.71 (0.22) 0.001 | 0.61 (0.30) 0.042 | 0.62 (0.33) 0.061 |
|
Note: *IMT was regressed on each radiation dose after adjusting for the effects of age and religion, **IMT was regressed on each radiation dose after adjusting for the effects of age, religion, FBS and HbA1c, #Adult doses=cumulative dose (lagged by 5 years)-pediatric dose, ##Both pediatric and adult doses were included in a regression model.
Table 8: Results of regression analyses for maximum IMT (raw data) according to the period of radiation exposure.
| Explanatory variable(s): Radiation dose | Regression coefficient (SE), mm Gy-1 P value |
|||
|---|---|---|---|---|
| All subjects (N=400) |
Subjects with FBS and HbA1c information (N=164) | |||
| Model 1* | Model 1* | Model 2** | ||
| Cumulative dose lagged by 5 years | 0.27 (0.09) 0.002 |
0.16 (0.13) 0.194 |
0.11 (0.13) 0.380 |
|
| Cumulative dose lagged by 10 years | 0.29 (0.10) 0.004 |
0.16 (0.15) 0.283 |
0.10 (0.15) 0.507 |
|
| Cumulative dose lagged by 15 years | 0.32 (0.12) 0.008 |
0.14 (0.17) 0.406 |
0.08 (0.18) 0.664 |
|
| Pediatric dose | 0.40 (0.25) 0.106 |
-0.05 (0.35) 0.887 |
-0.02 (0.35) 0.959 |
|
| Adult dose# | 0.41 (0.12) 0.001 |
0.29 (0.16) 0.078 |
0.21 (0.17) 0.227 |
|
| Pediatric dose and Adult dose## |
Pediatric dose | -0.19 (0.31) 0.548 |
-0.46 (0.39) 0.243 |
-0.36 (0.41) 0.385 |
| Adult dose | 0.47 (0.16) 0.003 |
0.40 (0.19) 0.035 |
0.31 (0.21) 0.138 |
|
Note: *IMT was regressed on each radiation dose after adjusting for the effects of age and religion, **IMT was regressed on each radiation dose after adjusting for the effects of age, religion, FBS and HbA1c, #Adult doses=cumulative dose (lagged by 5 years)-pediatric dose, ##Both pediatric and adult doses were included in a regression model, $Corrected maximum values of IMT after excluding IMT measurements over 2.5 mm.
Table 9: Results of regression analyses for maximum IMT (corrected data$) according to the period of radiation exposure.
In a regression model when adjusting for age and religion, a statistically significant association of mean and maximum IMT with radiation was found. The strongest association was with adult dose. This association with both IMT indexes became stronger when paediatric dose was also taken into account. In a further analysis in which potential confounding factors such as FBS and HbA1c were adjusted, adult dose was statistically significantly related to only raw mean IMT [regression coefficient (SE):0.28(0.10), P=0.008] and corrected mean IMT (regression coefficient (SE):0.24(0.10), P=0.018. Raw and corrected maximum IMT values were also related to adult doses. However, the associations were not statistically significant (raw maximum IMT, P=0.061 and corrected maximum IMT, P=0.138).
The increment of IMT per radiation dose observed in the present study was one order larger than the estimates obtained from a recent AHS of atomic bomb survivors [14,15]. A major difference between the subjects of the two studies is the nutritional conditions at the time of exposure. While radiation exposure of atomic bomb survivors took place at the time when they were in the status of under-nutrition, the study subjects of the present study were not in such a condition. Note that adult exposure was more important than paediatric exposure in the present study, and that the hyperlipidemia is more frequently observed among adults than among children. Another possibility is the difference in dose rates in the two studies. While atomic survivors were exposed to radiation in less than 1 second, Karunagappally women had chronic radiation exposure. At this moment, a paucity of our scientific knowledge on the biological effects of radiation dose rate cannot support or completely preclude such a hypothesis.
One may argue that the association observed in the present study can be limited to the people with hyperlipidemia even if the association is a causal one since Kerala is known to have a high prevalence of dyslipidemia [21]. Certainly, serum LDL cholesterol levels observed in this study suggest a high prevalence of hyperlipidemia in this population. The argument is supported by the absence of such an association in the cohort of atomic bomb survivors, in which the prevalence of hyperlipidemia is not high [15,16]. It should be pointed out, however, that the association was found in a study of HNBR area residents in Yangjiang, China, which is not known to have a high prevalence of hyperlipidemia [22].
One of the major limitations of the present study is the fact that 41% (164/400) of the subjects underwent biochemical tests to determine FBS and HbA1c, which are important potential confounding factors. When corrected maximum and mean IMT were regressed on adult dose lagged by 5 years, the exclusion of subjects without information on FBS decreased the regression coefficients from 0.41 to 0.29 (Table 7), and from 0.26 to 0.18 (Table 9), respectively. It is difficult to tell whether the confounding effects of FBS and HbA1c are that large in the rest of the study subjects, for which FBS or HbA1c data were not available. However, when we compared the health and socioeconomic status of the population which underwent FBS and HbA1c examinations with the rest of the population, they had similar characteristics. Therefore, we may assume that the women who received the HbA1c test are not particularly biased.
In the present study of women living in high natural radiation areas in south India, cumulative radiation exposure in adulthood showed a significant positive association with mean IMT value. After adjusting the effects of fasting blood sugar and HbA1c, the association between adulthood radiation exposure and mean IMT value remained statistically significant. Cumulative radiation exposure in adulthood showed a positive association with maximum IMT value without statistically significance. The association between radiation exposure in adulthood and mean/ maximum IMT values became stronger when paediatric radiation dose was adjusted. Cumulative radiation exposure in childhood was not related to IMT thickening. Further studies are necessary to confirm the findings observed in this study.
Meetings of the Institutional Scientific Review Board and Ethics Committee of Regional Cancer Centre on 12th December 2012 and 14th April 2013 approved the study, and all subjects gave written informed consent to participate as well. The study has also received approval from the ethics committee of Kagoshima University Graduate School of Medical and Dental Sciences. All procedures were carried out in accordance with the relevant guidelines and regulations of the above-mentioned boards
The written informed consent was obtained for publication from all the participants.
Athira Nandakumar: Conceived the study design and promptly initiated the investigation, oversaw the necessary procedures and data collection, and is responsible for the data analysis, proper interpretation of results, carefully drafted and finalized the manuscript; Ananthakrishnakurup Sreekumar: Conceived and efficiently carried out the research and also is responsible for the diagnostic interpretation of results and finalizing the manuscript; Abhilash TP: Performed the clinical examination, diagnostic interpretation and finalized the manuscript; Jayalekshmi Padmavathy Amma: Conceived the concept, designed the study, obtained funding, oversaw the data collection, and was responsible for the interpretation of results, drafting and finalizing the manuscript; Riyaz Ahammed: Performed the clinical examination, diagnostic interpretation, and involvement in finalizing the manuscript; Rekha A Nair: Responsible for the clinical data interpretation of results and finalizing the manuscript; Raghu Ram K Nair: Conceived the concept, designed the study, and is responsible for the interpretation of results, drafting and finalizing the manuscript; Chihaya Koriyama: Overviewed the data quality and data analysis and also a major contributor in the interpretation of the results and the manuscript preparation; Michiya Sasaki: Is responsible for the study design and data analysis; Suminori Akiba: Conceived the concept, designed the study, obtained funding, and is responsible for the data analysis, interpretation of its results, drafting and finalizing the manuscript; Seiichi Nakamura: Responsible for the interpretation of results, drafting and finalizing the manuscript; Junji Konishi and Keigo Endo: Responsible for the interpretation of results, drafting and finalizing the manuscript. All authors read and unanimously approved the final manuscript.
This study was supported by the Health Research Foundation, Japan through the Low-dose Radiation Research Centre of Central Research Institute of Electric Power Industry. CRIEPI had no role in study design, data collection, analysis, or interpretation. We acknowledge the support of Paul Sebastian, former director of Regional Cancer Centre Thiruvananthapuram and Dr. Ramachandran and his colleagues for the technical assistance for conducting the radio diagnosis part of this study. Dr. Padmanabhan Nair, Doctors Diagnostic Research Centre, we greatly acknowledge him for conducting the biochemical assays. The study was successfully completed with the wholehearted support of field enumerators of the Natural Background Radiation Registry, technicians and nursing staff members of the Cancer Care Centre. We also acknowledge the support of all study subjects.
This study was supported by the Health Research Foundation, Japan through the Low-dose Radiation Research Centre of Central Research Institute of Electric Power Industry.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Nandakumar A, Sreekumar A, Abhilash TP, Jayalekshmi PA, Riyaz A, Rekha AN, et al (2022) Natural Radiation Exposure and Carotid Intimamedia Thickness Among Women in Karunagappally, Kerala, India. Angiol Open Access.10:294.
Received: 24-Nov-2022, Manuscript No. AOA-22-20405; Editor assigned: 28-Nov-2022, Pre QC No. AOA-22-20405 (PQ); Reviewed: 12-Dec-2022, QC No. AOA-22-20405; Revised: 19-Dec-2022, Manuscript No. AOA-22-20405 (R); Published: 26-Dec-2022 , DOI: 10.35248/2329-9495.22.10.294
Copyright: © 2022 Nandakumar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.