International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
+44 1300 500008
ISSN: 2329-9096
+44 1300 500008
Short Communication - (2021)Volume 9, Issue 2
Near-Infrared Spectroscopy (NIRS) is well-established as a safe and effective monitoring tool for stroke recovery, including upper limb, lower limb recovery, motor learning, cortical function recovery, cerebral hemodynamic changes, cerebral oxygenation, therapy, clinical researches and evaluation of the risk for stroke. NIRS principles have been used to study brain oxygenation for several decades, but have more recently been applied to various uses. We provide a brief overview of our research and clinical applications of NIRS in the Physical Medicine and Rehabilitation field.
Aerobic exercise; Early mobilization; IoMT system
Near-infrared spectroscopy (NIRS) is a non-invasive method of measuring blood changes in brain tissue [1]. NIRS detects near- infrared light emitted from above the scalp and is reflected and scattered, and measures the change in absorbance. NIRS evaluates the relative changes in blood flow in the cerebral cortex located between the irradiation site and the measurement site. The NIRS device is small with fewer restrictions on the measurement environment than functional magnetic resonance imaging and positron emission tomography, although it has technical limitations such as difficulty in deep measurement and low spatial resolution as a brain function imaging technology. Moreover, our research uses a 2-ch NIRS, which is a small and light device (approximately 100 g). The application examples of NIRS rehabilitation that have been implemented so far are described.
According to the guidelines of the American College of Sports Medicine (ACSM), all healthy adults aged 18–65 years should participate in moderate-intensity aerobic physical activity for a minimum of 30 minutes for 5 days each week, or vigorous-intensity aerobic activity for a minimum of 20 minutes for 3 days each week. Aerobic exercise can be expected to improve the function of the respiratory and circulatory system in addition to burning body fat, leading to the prevention and improvement of hyperglycemia, dyslipidemia, hypertension, and arteriosclerosis. Aerobic exercise is beneficial for many conditions such as spinal cord injury [2], hemodialysis [3], rheumatoid arthritis [4], depression [5], cancer [6], osteoporosis [7], and multiple sclerosis [8]; there is also no exception for stroke patients [9,10]. Recently, attention has been paid to the immediate effect of aerobic exercise on higher brain function. Our group also confirmed a short-term improvement in working memory in the elderly in 2013 [11]. However, it is not clear whether this remains the case in post-stroke patients. Therefore, we examined the acute effect of physical exercise on prefrontal cortex activity in post-stroke patients using NIRS.
We studied 11 post-stroke patients (seven men and four women, with a mean age of 69.6 ± 12.0 years). Six patients had cerebral hemorrhage, while five had cerebral infarction. Three patients exhibited right hemiparesis, and eight patients exhibited left hemiparesis. The protocol was the same as that previously investigated in healthy adults. The patients performed Sternberg- type working memory tasks before and after moderate-intensity aerobic exercise (40% of maximal oxygen uptake) with an ergometer or a treadmill for 15 minutes. The subjects performed the Sternberg test 15 minutes after the end of the exercise. We measured concentration changes of oxyhemoglobin (oxy-Hb), deoxyhemoglobin (deoxy-Hb), and total Hb (t-Hb) in the bilateral prefrontal cortex (PFC) during the working memory task using a two-channel NIRS (PNIRS-10, Hamamatsu Photonics K.K., Japan). NIRS analysis revealed that physical exercise enhanced PFC activation, particularly the right PFC (p<0.05), during the working memory task compared with before the physical exercise. Interestingly, both accuracy and reaction time in the Sternberg test improved after physical exercise (p<0.01). Our results indicate that the introduction of moderate-intensity aerobic exercise into physical therapy improves not only motor ability but also cognitive performance in post-stroke patients by enhancing PFC activity during cognitive function [12]. In addition, NIRS showed that physical exercise increased the concentration changes of oxy-Hb in the PFC during the working memory task compared with the control condition, indicating that the improved performance of the working memory task was not caused by adaptation. We believe that moderate-intensity exercise enhanced PFC activity concomitantly with the improvement of working memory performance. Since our report, many studies have been conducted on the effects of aerobic exercise on stroke patients [13-15], and we would like to pay more attention to it.
Recently, rehabilitation from the acute phase, which is performed in parallel with emergency medical care, has attracted international attention. The guidelines of the American Society of Clinical Care Medicine (SCCM) [16] and the expert consensus of early rehabilitation of the Japanese Society of Intensive Care Medicine [17] recommend rehabilitation from the hyperacute stage. However, many guidelines do not provide consensus on the mobilization [18-20].
Delayed cerebral ischemia is more likely to occur in patients after subarachnoid hemorrhage (SAH), usually within the first 2 weeks after the bleeding. Therefore, the previous classical management was 2 weeks of absolute rest. Recently, many reports of early mobilization SAH patients have been studied [21,22]. Our group also reported affirmative results (Figure 1) [23]. However, its safety has not been sufficiently verified. In the present study, we investigated the relationship between cerebral cortex oxygenation and systemic circulation in the mobilization of patients with SAH using NIRS. Four patients with SAH were placed in a supine position for 5 minutes before mobilization, and every 5 minutes, they were tilted to 30°, 45°, and 60° positions using a tilt-table bed. Blood pressure was measured before and immediately after mobilization. We evaluated O2Hb levels in the left and right PFC in 11 of the 12 trials that could be performed without stopping because of orthostatic hypotension. We analyzed O2Hb levels at up to 60 seconds at 10-second intervals after mobilization from the mean 60 seconds before mobilization. The results showed that the O2Hb levels significantly decreased after 10 seconds (p<0.05) but returned to baseline after 20 seconds (Figure 2). The O2Hb level was higher than the baseline level before mobilization after 20 seconds to 60 seconds. In addition, blood pressure decreased by 6% on average across all 12 times. These results suggest that mobilization caused an instantaneous decrease in oxygenation associated with a decrease in the blood pressure; however, shortly afterward, it returned to baseline. It is especially important to monitor immediately after mobilization and recommend gradual mobilization of SAH patients within 2 weeks.
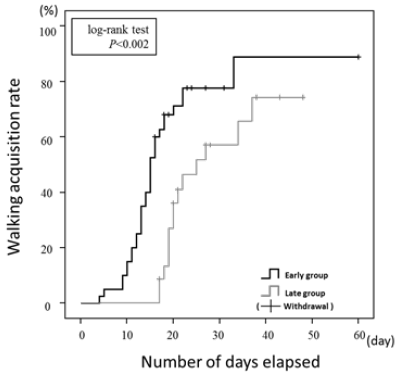
Figure 1:Kaplan-Meier survival curves of up to walking acquisition of subarachnoid hemorrhage patients. The walking acquisition rate was significantly higher in the Early group than in the Late group (log-rank test p<0.002).
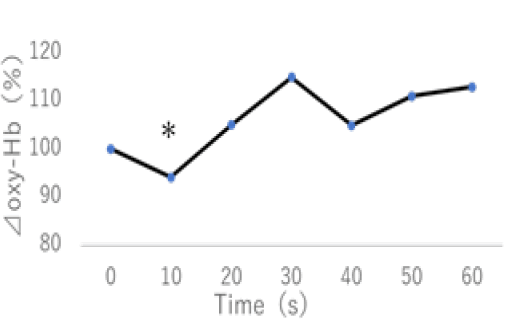
Figure 2:Change of Oxy-Hb by the mobilization. The Oxy-Hb levels significantly decreased after 10 seconds (p <0.05), but it returned to baseline after 20 seconds.
The Internet of Medical Things (IoMT) System is believed to be the next revolutionary technology and brings great benefits to various domains of society, including health care [24]. IoMT systems have gained increasing attention due to the development of solutions for tele-monitoring [25] and tele-treatment [26]. In our previous study, we developed an IoT-based health monitoring system, which allows for the long-term measurement of Blood Pressure (BP), Pulse Rate (PR), Body Weight (BW), and even mental stress measured from PFC activity using a wearable NIRS device [27]. The IoMT health monitoring system was set up in a fitness gym. The protocol is that, after instruction on how to use the IoMT system, the subjects were requested to monitor their physiological conditions by themselves when they visited the fitness gym. The objective health measurements included BP, PR, and BW. In addition, the HOT-1000 is a two-channel NIRS, which uses a single LED (800 nm) for measurements of total hemoglobin (t-Hb) concentration changes [28] (Figure 3). These devices have a Bluetooth communication function so that the collected data can be transported to the tablet receiver automatically. Data collected in receiver were analyzed at a remote University of Tokyo Graduate School. A total of 1392 measurements in 119 cases were possible for non-medical persons, of which 47 continued for 6 months. We found that the NIRS demonstrated that laterality index at rest (LIR) changed to positive values (left-dominant with low-stress responses) from negative values (right-dominant with high-stress responses) after exercise (p<0.01). There was a weak but statistically significant positive correlation between LIR and SBP (r=0.1, p<0.01) and LIR and MBP (r=0.1, p<0.05) (Figure 4). This indicates that the greater the right PFC activity at rest, the higher the BP. It should be emphasized that the present study is the first implementation of functional brain monitoring by NIRS. These findings suggest that ordinary people can use NIRS to monitor brain function and manage brain health by themselves.
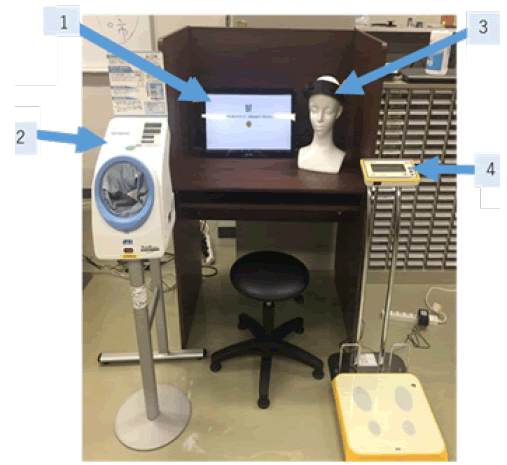
Figure 3: IoMT system. 1 Control PC, 2 Sphygmomanometer(TM-2657, A&D Corp.), 3 Two-channel-NIRS (HOT-1000, Hitachi High-Technologies Corporation), 4 Weight Scale (AD-6209PBT-C, A&D Corp.)
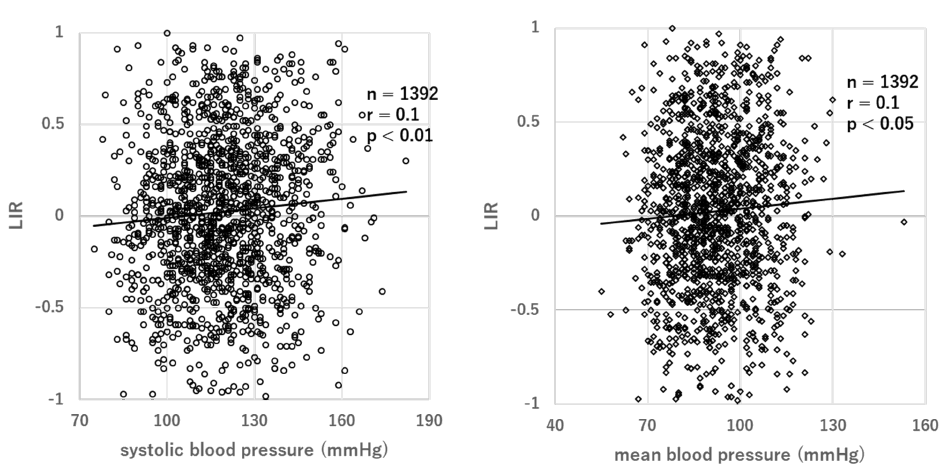
Figure 4: Correlation between LIR and SBP, LIR and MBP. Left-Right asymmetry was evaluated from the resting laterality index at rest (LIR) of each subject. To analyze the left/right asymmetry of PFC hemodynamic changes at rest, we calculated the LIR as follows:
Σt{(ΔoxyRt-ΔoxyRmin)-(ΔoxyLt−ΔoxyLmin)}/Σt{(ΔoxyRt-ΔoxyRmin)+(ΔoxyLt−ΔoxyLmin)}
A positive LIR indicates that (on average) the right PFC is more active at rest than the left PFC, while a negative LIR indicates that (on average) the left PFC is more active at rest than the right PFC.
NIRS is an imaging tool that has the advantage of measuring cortical activation without onerous constraints under daily life circumstances and in hospitals. Although NIRS is very often used in studies in the chronic phase, it is also used in the areas of intensive care and preventive physiotherapy as introduced here. Task-evoked changes in the skin blood volume induce artifacts in fNIRS signals. Collectively, fNIRS is a useful device for clinical physiotherapy and rehabilitation research.
The authors declare no conflicts of interest associated with this manuscript.
This research was supported by the Promotion and Mutual Aid Corporation for. Private Schools of Japan and Teikyo Heisei University Research Encouragement Grant (No.19TH013).
Citation: Moriya M, Satakani K (2021) Near Infrared Spectroscopy (NIRS) in Physical Medicine and Rehabilitation. Int J Phys Med Rehabil. 8:586.
Received: 06-Jan-2021 Accepted: 20-Jan-2021 Published: 27-Jan-2021 , DOI: 10.35248/2329-9096.21.9.586
Copyright: © 2021 Moriya M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This research was supported by the Promotion and Mutual Aid Corporation for. Private Schools of Japan and Teikyo Heisei University Research Encouragement Grant (No.19TH013).