Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Research Article - (2022)Volume 12, Issue 4
COVID-19-related pain and muscular weakness have been documented often in more severe instances and in post- mortem biopsies. Patients infected with the severe acute respiratory syndrome virus (SARS-CoV-2) have also suffered neurological damages. Furthermore, during and after an infection, immune-mediated necrotizing myopathy might develop. SARS-CoV-2 infection has also been linked to immune-mediated necrotizing myopathy in case reports. In this report, a young patient with minimal comorbidities was brought to the hospital with seronegative immune- mediated necrotizing myopathy due to COVID-19.
SARS-CoV-2; Necrotizing myopathy; Neuromuscular diseases
Inflammatory muscle disorders are a diverse autoimmune category defined clinically by muscular weakness caused by inflammation of the striated muscles. Cases of inflammatory myopathy are uncommon, and diagnosis can be difficult due to several subtypes of the disease, as well as the need for sophisticated laboratory testing [1,2].
The clinical history, time of illness progression, pattern of muscle involvement, and blood levels of muscle enzymes such as creatine phosphokinase, transaminases, aldolases, and troponin are used to make the diagnosis. Muscle biopsy is required for diagnosis, and magnetic resonance imaging scans can be used as a supplement. Although electroneuromyography data are not part of the formal diagnostic criteria, they might also be employed [3].
Immune-mediated necrotizing myopathy accounts for approximately 19% of all inflammatory myopathies and can develop at any age, but most commonly in adults. It is distinguished by muscle fiber necrosis with no or minor lymphocytic infiltration. The disease can take from days to weeks to progress, accompanied by severe weakness and high levels of the CK enzyme. Immune-mediated necrotizing myopathy can be brought on by viral infections, neoplastic processes, scleroderma, or the administration of statins [4].
The presence of autoantibodies has proved support in the diagnosis and classification of subgroups. Anti-Signal Recognition Particle (SRP) autoantibodies have a poor response to corticosteroid treatment and a poor prognosis. Patients with anti-HMGCR autoantibodies (HMG-CoA reductase) have a better prognosis and respond well to corticosteroids [5].
There is also a subgroup of myopathies that does not have autoantibodies. Information about therapy and the clinical course of seronegative immune-mediated necrotizing myopathy cases is limited. Despite the increased likelihood of cardiac problems, individuals with seronegative immune-mediated necrotizing myopathy have a better prognosis than those with seropositive myopathy [5].
Cases of SARS-CoV-2 infection and immune-mediated necrotizing myopathy have been reported in the literature [6-8]. A patient with seronegative immune-mediated necrotizing myopathy was documented in this case report using laboratory, histological, and clinical examinations.
This case report has ethical approval whose number is CAAE:32382820.3.0000.5256 and a signed consent form for participation in the patient's case report, without patient conflict of interest.
The patient was a 22-year-old woman, who had recently started taking oral contraception and had no medical conditions other than SARS-CoV-2 infection. Previous symptoms of diarrhoea and muscular pain were noted before and after the first dose of the vaccine against SARS-CoV-2 (AstraZeneca). The main medical hypothesis is that patient was immunized during a SARS-CoV-2 infection. Ten days before admission, she presented asthenia. Prednisone 40 mg/day and amitriptyline were administered with no improvement in her condition. Upon admission, the patient had tetraparesis, with strength grade 0 in the lower limbs, grade 1 in the right upper limb, and grade 2 in the left upper limb.
Laboratory tests on admission showed Table 1 an increase in Aspartate Transaminase (AST) 972 U/L, Alanine Transaminase (ALT) 438 U/L, and Lactate Dehydrogenase (LDH) 1026 U/L and no troponin positivity, confirming myositis. During hospitalization, Creatine Kinase (CK) dosage reached 46121 U/L. The patient never showed any signs of renal dysfunction or rhabdomyolysis, which are common in critically ill patients with coronavirus infection and who are on mechanical ventilation.
| Clinical parameters | Day 0 | Day 1 | Day 2 | Day 3 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 12 | Day 13 | Day 14 | Day 15 | Day 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematocrit (women 35%-45%) | 46.4 | 43.1 | 41.9 | 39.7 | 38 | 39.2 | 35.2 | 32.6 | 41.9 | 26.6 | 24.4 | 27.2 | 29.1 | 26.3 |
| Hemoglobin (15.7 ± 1.7 g/dL) | 15.1 | 13.8 | 13.6 | 13.1 | 12 | 12.5 | 11.2 | 10.4 | 13.63 | 8.3 | 7.8 | 8.7 | 9 | 8.6 |
| Leukocytes (4000-10000 mm3) | 10200 | 8400 | 7600 | 7100 | 9200 | 8900 | 7700 | 8300 | 7600 | 9200 | 8200 | 11600 | 14000 | 5500 |
| Lymphocytes (20-50%) | 20 | 19 | 32 | 13 | 13 | 20 | 9 | 7 | 10 | 9 | 14 | 12 | ||
| Monocytes (2-10%) | 14 | 7 | 13 | 9 | 4 | 2 | 7 | 6 | 9 | 9 | ||||
| Platelet count (150000/-450000/mm³) | 142000 | 132000 | 113000 | 105000 | 120000 | 112000 | 116000 | 157000 | 113000 | 258000 | 205000 | 297000 | 406000 | 158000 |
| CKMB ( until 5.0 ng/mL) | 167.2 | 151.2 | 127.5 | 140.8 | 78.4 | 78.4 | 168.3 | 127.5 | 17.8 | 20.3 | 95.9 | - | 13 | |
| Myoglobin (women 7-64 ng/mL) | >900 | >900 | >900 | |||||||||||
| Troponin (until 0.018 ng/mL) | 0.008 | 0.197 | 0.0276 | 0.015 | 0.013 | - | 0.013 | 0.009 | 0.026 | 0.006 | 0.008 | - | - | 0.005 |
| D-Dimer (500 ng/mL) | 2780 | 2820 | 2780 | 4390 | 3720 | |||||||||
| AST (women until 32 U/L) | 972 | 1049 | 983 | 665 | 1039 | 1265 | 292 | 483 | 669 | 114 | ||||
| ALT (women until 31 U/L) | 438 | 366 | 276 | 280 | 265 | 273 | 273 | 179 | 189 | 289 | 133 | |||
| CPK (women >145 U/L) | 18203 | 38434 | 39263 | 27873 | 26120 | 46121 | 29757 | 7459 | 30602 | 1416 | ||||
| PCR mg/dL* | 2.8 | 1.52 | 1.94 | 2.1 | 2.75 | 5.83 | 1.58 | 2.6 | 3 | 2 | 2.13 | |||
| Urea (10-45 mg/dL) | 29.5 | 25 | 19 | 27 | 25 | 27 | 20 | 29 | 19,3 | 18 | 27 | 18 | ||
| Blood Glucose Level (under 99 mg/dL) | 85 | 96 | 80 | 84 | 96 | 102 | 144.7 | 90 | 128 | 146 |
Note: * PCR (1.0-5.0 mg/dL: viral infections and mild inflammatory processes; 5.1-20.0 mg/dL: bacterial infections and systemic inflammatory processes; over 20.0 mg/dL: serious infections, major burns and multiple trauma).
Table 1: Clinical parameters in laboratory tests for confirming myositis.
The pelvic girdle and scapula had significant muscle edema, as revealed by magnetic resonance imaging. Transthoracic echocardiography revealed no involvement of the cardiac muscles. However, there were abnormalities in the esophageal transit scintigraphy, which was unusually lethargic, with a time of 85 seconds (the average is less than 12 seconds). In addition, changes in the patient’s visual acuity were observed, as well as edema in the chest wall, revealing significant systemic inflammation.
Fundoscopy revealed lesions suggestive of multifocal retinitis and intraretinal hemorrhage with a blurring point near the arches Figure 1. Biomicroscopy remained unaltered during evolution, but the fundoscopic examination showed mild arteriolar constriction, increased vascular tortuosity, persistence of yellowish lesions suggestive of multifocal retinitis, and increased cotton-wool exudate containing the intraretinal hemorrhages previously seen. Intraretinal cysts were also reported, with worsening compared to the previous exam, as well as serous retinal detachment, and an increase in nerve fiber layer thickness. These alterations were thought to be a result of severe systemic inflammation.
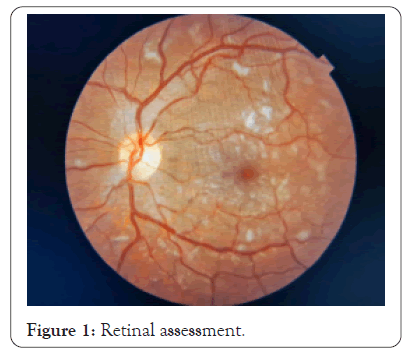
Figure 1: Retinal assessment.
The main autoantibodies associated with inflammatory myopathies and rheumatologic diseases were measured during hospitalization: anti-RF, anti-CCP, anti-RO, anti-La, anti-SM, anti-RNP, anti-DNA, anti-mitochondria, anti-Jo1, anti-Mi2, anti-SRP, and anti-HMG- Coa but none of them were detected. The dosage of antinuclear antibodies of 1:80 showed a dense speckled nuclear pattern and a normal complement. Viral serologies for detection of HCC, HAV, HBV, HIV, and HTLV were negative. However, positive rapid viral antigen tests and RT-PCR for SARS-CoV-2 were reported.
The diagnosis was concluded mainly from the result of the biopsy of the vastus lateralis muscle Figure 2, which shows little inflammatory infiltrate and sparse fiber necrosis, consistent with immune-mediated necrotizing myopathy.
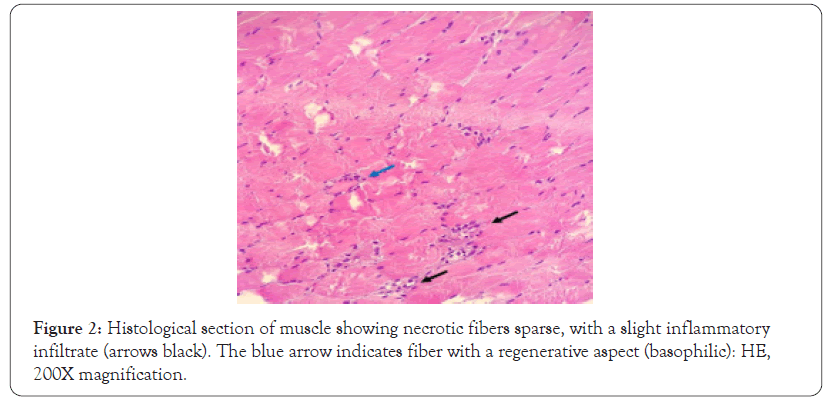
Figure 2: Histological section of muscle showing necrotic fibers sparse, with a slight inflammatory infiltrate (arrows black). The blue arrow indicates fiber with a regenerative aspect (basophilic): HE, 200X magnification.
Thus, the diagnostic hypothesis for the young woman's immune- mediated necrotizing myopathy was that it was caused by the coronavirus infection, which acted directly or indirectly on the striated musculature.
The treatment was started with pulse therapy with 1 g/day of methylprednisolone for five days, but there was no improvement in the muscle edema, without muscle strength recovery, nor laboratory test normalization. Then, intravenous human gamma globulin, 27 g/day, was administered for five days, along with azathioprine and oral hydroxychloroquine. The new therapeutic scheme showed progressive improvement in muscle strength and edema with a consequent decrease in muscle enzyme levels. The quantification of the pro-inflammatory cytokines was within the expected reference values.
The patient was discharged from the hospital with improved muscle symptoms but no postural control or ambulation, with a maintenance phase of therapy that included monthly immunoglobulin infusions for two months, slow weaning of oral corticosteroids, and continued low-dose azathioprine.
Five months after hospitalization, the patient remains under clinical follow-up and practices physical activities. She maintains no positive autoantibodies for diagnosis of rheumatologic disease and the only sequelae of the infection that remains is the alteration in the fundoscopy exam, with the presence of telangiectasias in the upper and temporal periphery superiority of the right eye and reduction of the vascular zone in the left eye.
Myositis in SARS-CoV-2 patients is not uncommon. It is has been seen in many critically ill patients who are on mechanical ventilation and have kidney injury and systemic involvement [9]. However, it is rare to see only severe musculoskeletal involvement [10]. In this case, coronavirus might be neurotropic, causing muscle damage directly or indirectly, with an increase in enzymes close to 50,000 and significant muscle edema, with a risk of compartment syndrome, as reported in this case [11].
Although cases of myopathy associated with SARS-CoV-2 infection usually manifest in patients with severe lung impairment and undergoing invasive procedures, Beydon [12] reported a case of myopathy in a patient with COVID-19 without respiratory symptoms or fever on admission. Here, a case was also observed in which the patient had no pulmonary symptoms attested by chest x-ray. However, what is noteworthy is that the CK levels were much higher than the values cited in most reports, and even so the patient did not have a renal function impairment or rhabdomyolysis [13,14].
Remarkably, the inflammation was not limited to skeletal muscles. Here, changes in visual acuity and slowed esophageal transit were observed. Although the primary symptoms were indeed muscular, the systemic inflammation caused damages to other organs, almost all of which are reversible with venous gamma globulin treatment. In myopathies, a muscle weakness is usually proximal. The eye muscles are spared. It can involve the extensor muscles of the neck and pharyngeal muscles, with head droop and dysphagia. In severe cases, it affects respiratory muscles, evolving to respiratory failure. The main extramuscular manifestations include fever, arthralgia, Raynaud's phenomenon, cardiac arrhythmias, and interstitial lung disease (10 to 40%) in the other myopathies subtypes [15]. Over the last 15 years, biopsies have shown that myopathies have significant muscle necrosis but with minimal inflammatory infiltrate. These patients are known to have immune-mediated Necrotizing Myopathy (NAM). The etiology is called idiopathic, if it is not associated with the use of statins, because it triggers a viral infection, as happens with infections by Influenza A and B, Epstein Barr virus, cytomegalovirus, HIV or being paraneoplastic [13,14,16].
Retrospective studies have shown patients with myalgia and muscle weakness with high levels of CK, suggesting possible immune- mediated myopathy. However, biopsies for diagnosis are rarely performed in cases of viral infections [2]. In the case reported here, a biopsy was performed to confirm the diagnosis of necrotizing myositis, which is responsible for the highest CK values found in the myositis group [16,17]. The histopathological report showed a small infiltrate, with some areas of necrosis, confirming the hypothesis. However, there was no correlation between the diagnosis and a specific autoantibody. The recent literature has already registered numerous cases of myopathy in the presence of coronavirus infections. A case-control study reported a series of patients who died from COVID-19 with signs of myopathy [6]. The publication of this clinical case, therefore, is important to register new forms of clinical manifestations resulting from COVID-19 and to increase the knowledge of health professionals about the infection. Another contribution is related to the treatment of similar cases, which do not respond to corticosteroids, but respond well to venous gamma globulin.
The diagnosis was concluded mainly from the result of the biopsy of the vastus lateralis muscle, which shows little inflammatory infiltrate and sparse fiber necrosis, consistent with immune- mediated necrotizing myopathy.
Thus, the diagnostic hypothesis for the young woman's immune- mediated necrotizing myopathy was that it was caused by the coronavirus infection, which acted directly or indirectly on the striated musculature.
The treatment was started with pulse therapy with 1 g/day of methylprednisolone for five days, but there was no improvement in the muscle edema, without muscle strength recovery, nor laboratory test normalization.
We would like to thank the Rear admiral Oscar Artur de Oliveira Passos and Capitan Marcelo Leal Gregorio for the support.
This work was supported by grants from the State of Rio de Janeiro Research Foundation- FAPERJ E-26/010.000168/2020.
The authors declare no conflicts of interests.
Citation: Nico D, Cardoso KM, Siqueira E, Barroso SPC, Pereira FCF, de Oliveira WCB, et al. (2022) Necrotizing Myopathy in SARS-CoV-2 Infection. Rheumatology (Sunnyvale). 12:311.
Received: 06-Jul-2022, Manuscript No. RCR-22-18242; Editor assigned: 11-Jul-2022, Pre QC No. RCR-22-18242 (PQ); Reviewed: 26-Jul-2022, QC No. RCR-22-18242; Revised: 02-Aug-2022, Manuscript No. RCR-22-18242 (R); Published: 08-Aug-2022 , DOI: 10.35841/2161-1149.22.12.311
Copyright: © 2022 Nico D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.