Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2020)Volume 11, Issue 4
Purpose: Examine the need and the timeframe to redo selective laser trabeculoplasty (SLT) that was applied in open
angle glaucoma or ocular hypertensive patients.
Methods: Patients received SLT as primary, adjunctive or replacement therapy. Data were recorded up to 5.5 years
after SLT treatment. Target pressure was defined as intraocular pressure at least 20% lowered. On exceeding the
target pressure, patients received a second SLT. Primary outcome were the need and the time to redo the SLT. We
examined differences between the groups (primary, replacement or adjunct SLT) and correlations between time and
need to redo and pre-SLT parameters.
Results: 108 patients (194 eyes) could be followed for at least 0.5 year and up to 4.5 years, with a mean follow up of
22.35 ± 18.94 months. Our population at start was a varied one; 34% of patients received primary SLT, 50% had
replacement SLT, 16% had SLT as adjunctive treatment. These three groups showed no difference in evolution of
IOP or medication in time. Time to redo varied, with a mean of 31.13 ± 11.24 months.
Conclusion: We set out to have a general idea of how many patients could be expected to need a retreatment with
SLT after a first successful SLT in a private clinic setting. In our population, the percentage of redo needed was 5.6%
after 2 years, 35.4% after 3 years and 45.4% after 4 years. No differences could be measured with regard to the type
of SLT performed nor could any significant correlation be found between need to redo and pre-SLT characteristics.
Selective laser trabeculoplasty; Long term; Retreatment
Glaucoma is the leading cause of irreversible blindness in the world. Population growth and ageing are expected to additionally increase the number of people affected by this chronic disease [1,2]. Currently, only lowering of the intraocular pressure (IOP) has proven to delay disease onset and slow down its progression [3,4]. Medication has been the first choice treatment for several years but recent studies provide arguments to prefer selective laser trabeculoplasty (SLT) [5,6].
Selective laser trabeculoplasty (SLT) has proven to be a valid alternative to treat intraocular pressure (IOP) in several large, randomized controlled trials. It is as effective as medication and as argon laser trabeculoplasty (ALT) in glaucoma and ocular hypertension patients. It can be used as primary, adjacent and as replacement therapy [5-9].
The mechanism by which SLT lowers IOP is probably not mechanical but rather a biochemical response of the trabecular meshwork tissue [10,11] with increased cell division improving outflow and the attraction of macrophages that clear up debris at the trabecular meshwork [9,12-14]. Enhanced secretion of chemokines and increased expression of matrix metalloproteinase is expected to mediate these responses [15,16].
The effect of selective laser trabeculoplasty however lowers in time [17-19]. Fortunately, SLT can be repeated with equal success after more than 6 months [20,21]. The success of this second SLT lasts as least as long as the primary treatment [21-24]. It is less clear however how long after initially successful SLT therapy this redo commonly is needed.
For instance in underdeveloped countries, SLT can make a huge difference. Medical therapy can be costly compared to income and surgery has limited availability, whereas SLT proves to be effective, safe, quick, easy to perform and cheap [25,26]. But when do we need to plan a retreatment?
To evaluate the actual time to redo and the number of patients that need retreatment after SLT, we set up an observational study. Primary outcome was the need for and the time it took to perform a second selective laser trabeculoplasty in order to keep IOP below target pressure without adding medication.
Study design and subjects
A prospective non-randomized observational non-comparative case series trial was conducted at Medipolis Eye Centre in Antwerp, Belgium. Patients were consecutively enrolled from July 2016 to December 2019.
Inclusion criteria concerned all kinds of open angle glaucoma (OAG), including normal tension glaucoma, and ocular hypertension (OHT). Patients were allowed with and without prior anti-glaucoma medication and with controlled or uncontrolled IOP.
Exclusion criteria were all corneal diseases that inhibited good visualization of the trabecular meshwork and the use of systemic steroids.
The study followed the Tenets of the Declaration of Helsinki and was ethically approved by our Institutional Board. Written informed consent was obtained from all participants. The trial was registered as NL6144.
Baseline examinations
At baseline a full ophthalmological examination of each study participant was conducted, including a medical history review. Best corrected visual acuity measurement was taken as well as IOP measurement using Goldmann applanation tonometry, slit lamp examination of the anterior segment (gonioscopy), central corneal thickness (CCT) measurement (Avanti Widefield OCT, Optovue Inc, Fremont, USA), dilated fundus examination, visual field examination by computerized perimetry (program 24-2, Humphrey Field Analyzer 3, Zeiss, Jena, Germany), optical coherence tomography (OCT)( (Avanti Widefield OCT, Optovue Inc, Fremont, USA) of the optic nerve head and recording of glaucoma medications.
We used focal loss of volume (FLV) as determinant for the OCT 27. IOP before treatment was calculated as the mean of three measurements taken on 3 different visits, each 4 to 6 months apart, before starting anti-glaucoma medication. IOP at baseline was calculated as the mean of the Goldman measurements made on different time points on the three last visits before laser treatment.
Laser technique
SLT was performed by the same experienced ophthalmologist (MDK). A frequency doubled, Q-switched Nd:YAG laser was used, emitting a wavelength of 532 nm, coupled to a slit lamp delivery system (Solo, Ellex Ltd, Adelaide, Australia). 100 non-overlapping laser applications were performed in 360° of the trabecular meshwork through a laser gonioscopy lens. The laser energy was initially set at 0,9 mJ and titrated to achieve minimal or static cavitation bubbles in about two out of five laser applications [5].
Immediately before the laser procedure a drop of pilocarpine 1% and apraclonidine 0.5% were instilled into the treated eye [27,28]. After the laser treatment no anti-inflammatory drops were administered [28,29].
Postoperative management
Patients were examined 1 week, 3 and 6 months after SLT, followed by a check-up every 6 months. IOP values were calculated as the average of 2 measurements by Goldmann applanation tonometry. After SLT, the previously used antiglaucoma drops were continued until IOP was more than 2 mmHg below target pressure, at which point they were stopped one by one.
Target pressure was calculated using the formula proposed by H. Jampel (Target IOP= maximum IOP – maximum IOP% - z, where z is an optic nerve damage severity factor).
Target pressure had to be at least 20% lower than IOP before treatment.
Patients that had insufficient IOP-lowering 3 and 6 months after selective laser trabeculoplasty were considered non-responders to SLT and left out of the final analysis.
A retreat SLT was defined as an SLT performed when a patient had an initially successful SLT after 3 and 6 months but had a rise of IOP>20% at later visits.
A successful selective laser trabeculoplasty was defined as an IOP below target pressure without medication for primary SLT. Success was an IOP below target pressure achieved with at least one medication less after the SLT than before, for replacement SLT, and for adjunctive SLT success was defined as IOP below target pressure without additional treatment needed.
Statistical methods
One-way Anova was used to examine differences in glaucomatous parameters between the groups that had different SLT performed. Post hoc analysis revealed several significant differences at start. A second one-way Anova and post hoc analysis were performed on the IOP measures and number of medications at different time points. A Pearson productmoment correlation was conducted to examine the relationships between time to redo and glaucomatous parameters at start (IOP pre-SLT, BCVA, CCT, cdr, VF, OCT). None of these parameters was correlated significantly to the time to redo. The same correlationtest was performed on the need to redo, but no significant correlation could be found.
A Kaplan-Meier analysis and hazard curve were made to examine the survival rate of the primary SLT.
Results of statistical analysis with p-values<0.05 were considered to be significant.
Population
133 patients (237 eyes) were enrolled in the study. 12 patients (21 eyes) could not be followed for longer than 6 months, they were considered drop-outs. 13 patients (22 eyes) (10.1%) did not show sufficient IOP lowering 3 and 6 months after SLT. They were considered non-responders. 108 patients (194 eyes) could be followed for at least 0.5 year and up to 5.5 years, with a mean follow up of 22.35 ± 18.94 months.
We had a mostly elderly population (69.5 y), 47.6% female and 93% Caucasian. Demographic characteristics can be found in Table 1.
| Demography | |
|---|---|
| Age | 69.5 ± 10.6 y |
| Sex | 47.6.0% ♀ |
| 52.4% ♂ | |
| Ethnicity | 93.0% Caucasian |
| 6.6% African | |
| 0.4% Asian | |
| Hypertension | 0.306 |
| Diabetes | 0.052 |
| Blood dilutors | 0.183 |
| Pseudophakic | 0.162 |
Table 1: Demography.
The largest group of our population were patients with open angle glaucoma (64.6%), followed by a substantial group of normal tension glaucoma patients (30.6%) and some patients with ocular hypertension (4.8%). 34.22% of our population was treatment naïve, 61.78% were already on anti-glaucoma medication pre-SLT, with a mean of one anti-glaucoma eye-drop pre-SLT treatment. Prostaglandin analogs were the most commonly used kind of drops (44.1%), followed by beta-blockers (29.7%).
Mean pre-SLT IOP was low (16.86 ± 4.97 mmHg); 44.00% of our patients had controlled IOP pre-SLT, the others (56.00%) had uncontrolled IOP with (17.78%) or without medication (34.22%). Other glaucomatous characteristics can be found in Table 2.
| Glaucoma at start | |
|---|---|
| Type | 64.6% OAG/30.6% NTG |
| 4.8% OHT | |
| Therapy pre-SLT | 34.6% no/65.4% drops |
| Function of SLT | 34% primary treatment |
| 50% replacement SLT | |
| 16% adjunctive SLT | |
| Eye drops # | 1.0 ± 1.0 |
| Eye drops | 44.1% prost/29.7% beta-bl |
| 22.1% CAI/6.6% alpha | |
| Pre-SLT IOP | 16.7 ± 5.0 mmHg |
| BCVA | 0.8 ± 0.3 |
| CCT | 539.6 ± 41.1 um |
| Cdr | 0.6 ± 0.3 |
| VF md | 6.3 ± 7.4 dB |
| OCT flv | 4.7 ± 5.2 |
Table 2: Glaucomatous characteristics. OAG: Open Angle Glaucoma; NTG: Normal-Tension Glaucoma; OHT: Ocular Hypertension; prost: Prostaglandin Analogs or Prostamides; beta-bl: Beta-Blockers; CAI: Carboanhydrase Inhibitors; alpha: Alpha-Agonist; BCVA: Best Corrected Visual Acuity; CCT: Central Corneal Thickness; Cdr: Cup Disc Ratio; VF md: Visual Field Mean Deviation; OCT flv: Optical Coherence Tomography Focal Loss of Volume.
Our population at start was a divers one; 34.22% of patients received SLT as primary treatment, 44.00% as a replacement for medication, 17.78% as adjunctive treatment.
IOP pre-SLT was higher in both the primary and adjunct SLT group and significantly different from pre-SLT IOP in the replacement group (p<.05). Central corneal thickness was significantly higher in the primary SLT group compared to the two other groups (p<.05). Cup-disc ratio, visual field mean deviation and OCT focal loss of volume were not significantly different between the three groups (p>.05). Best corrected vision, the number of medications and the% of prostaglandins used pre-SLT were comparable between the replacement and the adjunct SLT group, but significantly different from the primary SLT group (p<.05) (Table 3).
| Primary SLT N=77 | Replacement SLT N=99 | Adjunctive SLT N=40 | P* | |
|---|---|---|---|---|
| Pre-SLT IOP (mmHg) | 17.60 ± 4.94 | 15.01 ± 3.97 | 19.95 ± 5.66 | 0.002* |
| POAG/NTG/OHT | 34/58/8% | 62/34/ 4% | 77/23/0% | <0.01* |
| BCVA | 0.91 ± 0.15 | 0.71 ± 0.32 | 0.78 ± 0.32 | 0.04* |
| CCT (um) | 549.60 ± 32.42 | 533.53 ± 44.90 | 536.11 ± 42.31 | 0.02* |
| Cdr | 0.59 ± 0.28 | 0.72 ± 0.28 | 0.63 ± 0.33 | 0.91 |
| VF md (dB) | 3.48 ± 2.64 | 7.68 ± 8.42 | 6.88 ± 8.17 | 0.7 |
| OCT flv | 2.73 ± 3.15 | 5.92 ± 5.44 | 5.20 ± 5.60 | 0.37 |
| # drops pre-SLT | 0 | 1.68 ± 1.03 | 1.71 ± 1.07 | <0.01* |
| % prostaglandin analogues | 0 | 0.7895 | 0.8462 | <0.01* |
Table 3: Glaucomatous characteristics of different SLT groups. BCVA: Best Corrected Visual Acuity, CCT: Central Corneal Thickness, cdr: Cup Disc Ratio, VF md: Visual Field Mean Deviation, OCT flv: Optical Coherence Tomography Focal Loss Of Volume, *p<.05 is considered significant.
Laser technique
All patients received a 360° treatment (Nagar) 31 of the trabecular meshwork, applied by the same experienced surgeon (MDK). We used a mean number of 101.9 ± 13.6 nonoverlapping spots with a mean energy of 1.4 ± 0.5 mJ. SLT was used as primary therapy in 34.22% of our patients, as a replacement therapy to anti-glaucoma drops in 44.00% of the patients. And as an adjunctive therapy in patients with uncontrolled IOP under medication in 17.78%.
Evolution of IOP
IOP lowered from a mean of 16.86 ± 4.97 mmHg at start to 13.10 ± 3.33 mmHg after 3 months and 13.79 ± 3.58 mmHg after 6 months (Table 4).
| Whole group | Primary SLT | Replacement SLT | Adjunctive SLT | ||
|---|---|---|---|---|---|
| IOP (mmHg)(n) | N=194 | N=72 | N=91 | N=31 | P* |
| Start | 16.86 ± 4.97 (194) | 17.53 ± 4.99 (72) | 15.13 ± 4.10 (91) | 20.32 ± 5.29 (31) | .00* |
| 3 m | 13.10 ± 3.33 (184) | 12.89 ± 3.43 (69) | 12.84 ± 2.93 (88) | 14.48 ± 4.06 (27) | 0.14 |
| 6 m | 13.79 ± 3.58 (163) | 13.86 ± 3.42 (58) | 13.65 ± 3.72 (81) | 14.08 ± 3.66 (24) | 0.96 |
| 12 m | 13.61 ± 3.56 (141) | 13.50 ± 3.84 (54) | 13.64 ± 3.54 (66) | 13.81 ± 3.08 (21) | 0.99 |
| 18 m | 14.03 ± 3.00 (101) | 13.63 ± 2.83 (35) | 13.94 ± 2.98 (53) | 15.46 ± 3.38 (13) | 0.3 |
| 24 m | 14.20 ± 2.87 (80) | 14.52 ± 2.68 (25) | 14.00 ± 2.84 (49) | 14.50 ± 4.37 (6) | 0.89 |
| 30 m | 14.96 ± 3.27 (53) | 14.94 ± 2.14 (16) | 14.80 ± 3.76 (35) | 18.00 ± 0.0 (2) | 0.63 |
| 36 m | 14.81 ± 3.23 (35) | 15.14 ± 3.39 (7) | 14.71 ± 3.31 (28) | 0.95 | |
| 42 m | 13.95 ± 2.90 (22) | 11.67 ± 1.15 (3) | 14.32 ± 3.02 (19) | 0.36 | |
| 48 m | 15.34 ± 3.63 (22) | 14.67 ± 4.62 (3) | 15.47 ± 3.9 (19) | 0.94 | |
| 54 m | 13.52 ± 3.56 (20) | 15.0 ± 0.0 (2) | 13.39 ± 3.82 (18) | 0.83 |
Table 4: Evolution of IOP in time. N, n= number, *p<.05 is considered significant.
IOP was significantly different between the groups at start; IOP was higher in the two groups with uncontrolled IOP pre-SLT. IOP in the primary SLT group was 17.53 ± 4.99 mmHg at start and in the adjunct SLT group 20.32 ± 5.29 mmHg, as opposed to 15.13 ± 4.1 mmHg in the replacement SLT group. Three months after SLT, the difference in IOP became smaller and was no longer significant (p .052). At no other time point there was a significant difference in IOP evolution between the three groups (p>.05).
We did record a high number of non-responders in the adjunct SLT group (22.5%- 9 eyes), compared to 6.5% (5 eyes) in het primary SLT and 8.1% (8 eyes) in the replacement SLT group (p<.01).
The use of medication dropped from a mean of 0.96 ± 0.98 pre- SLT to 0.78 ± 1.05 and 0.43 ± 0.84 at 3 and 6 months respectively. At no time point there was a significant difference in medication use between the replacement and adjunctive SLT group. In the primary SLT treatment group no medication was used at any time point (Table 5).
| Whole group | Replacement SLT | Adjunctive SLT | ||
|---|---|---|---|---|
| # med (n) | N=194 | N=91 | N=31 | P* |
| start | 0.96 ± 0.98 (194) | 1.51 ± 0.84 (91) | 1.58 ± 0.72 (31) | 0.84 |
| 3 m | 0.78 ± 1.05 (192) | 1.19 ± 1.12 (91) | 1.45 ± 0.95 (29) | 0.32 |
| 6 m | 0.43 ± 0.84 (168) | 0.69 ± 0.97 (81) | 0.62 ± 0.98 (26) | 0.94 |
| 12 m | 0.32 ± 0.70 (141) | 0.47 ± 0.68 (66) | 0.67 ± 1.1 (21) | 0.42 |
| 18 m | 0.23 ± 0.63 (101) | 0.32 ± 0.64 (53) | 0.46 ± 1.13 (13) | 0.74 |
| 24 m | 0.19 ± 0.56 (88) | 0.35 ± 0.72 (49) | 0.0 ± 0.0 (10) | 0.16 |
| 30 m | 0.25 ± 0.62 (53) | 0.37 ± 0.73 (35) | 0.0 ± 0.0 (2) | 0.68 |
| 36 m | 0.43 ± 0.79 (35) | 0.54 ± 0.79 (28) | ||
| 42 m | 0.32 ± 0.57 (22) | 0.37 ± 0.60 (19) | ||
| 48 m | 0.23 ± 0.53 (22) | 0.26 ± 0.56 (19) | ||
| 54 m | 0.20 ± 0.52 (20) | 0.33 ± 0.59 (18) |
Table 5: Use of medication in time. N, n= number, *p<.05 is considered significant.
Need and time to redo
A total of 23 eyes (11.86%) needed a second SLT; 5 eyes in the primary SLT group, 18 in the replacement group, none in the adjunctive SLT group. We made no separate analysis per SLT group because these numbers were too small. The Kaplan-Meier survival curve demonstrated a steep change in survival around 30 months (Figure 1). The mean time to redo was 31.13 ± 11.24 months. This is reflected in the hazard function curve (Figure 2).
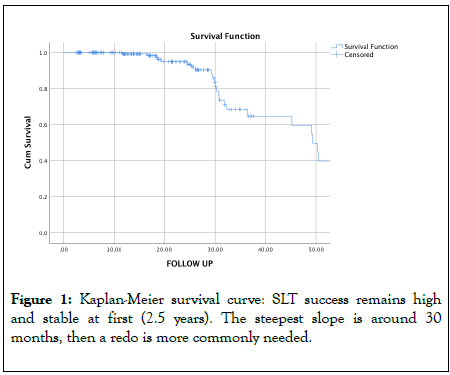
Figure 1: Kaplan-Meier survival curve: SLT success remains high and stable at first (2.5 years). The steepest slope is around 30 months, then a redo is more commonly needed.
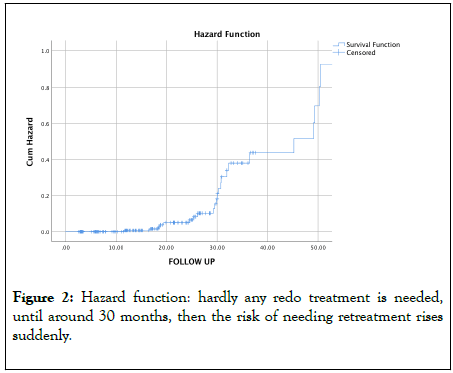
Figure 2: Hazard function: hardly any redo treatment is needed, until around 30 months, then the risk of needing retreatment rises suddenly.
99% of our treated eyes still had IOP below target pressure after one year, 93.5% after 2 years, 81.0% after 2.5 years, 64.6% after 3 years, 59.6% after 3.5 years and 54.6% after 4 years.
One year after successful SLT only 0.7% of our patients needed a redo. At 1.5 years 0.9%, 1% equally at 1.5 years, 5.6% at 2 years and 7.0% at 2.5 years. At their 3-year control, 20.5% of the patients had retreatment with SLT, 33.3% after 3.5 years, 28.6% after 4 years and 52.5% of patients that had long enough follow up needed redo after 4.5 years. The difference became significant after 2.5 years.
Correcting for differences in follow up time, the Kaplan-Meier survival analysis predicted a need to redo of 1.0% one year after initial SLT. At 1.5 years 2.8%, 6.05% at 2 years, 6.19% at 2.5 years, 35.4% would need redo after 3 year, 40.4% after 3.5 years and 45.4% after 4.5 years. The biggest step up was after 30 months.
A Pearson product-moment correlation was conducted to examine the relationships between the time to redo and the glaucomatous parameters at start (IOP pre-SLT, BCVA, CCT, cdr, VF, OCT). None of these parameters was correlated significantly to the time to redo. The same correlation tests were performed on the need to redo, but no significant correlation could be found with either glaucoma parameter.
Keeping in mind that we eliminated the non-responders from our series and incorporated only the successful SLT treatments into further analysis, our patients had a one year survival rate of 99.0%, 93.5% after 2 years, 81.0% after 2.5 years, 64.6% after 3 years, 59.6% after 3.5 years and 54.6% after 4 years.
It was also unexpected that no (0) eyes needed a redo treatment in the replacement group. Since the mean time to redo was 31.23 months, this may be the result of the limited follow up (30 months) in this group.
SLT has proven to be an effective therapy for the treatment of glaucoma and ocular hypertension [5,7-9]. Success of an SLT treatment only seems influenced by high baseline IOP [32-34]. There do seem to be racial differences in SLT efficacy [26] and the effect of using prostaglandins prior to SLT treatment [22,33] is debated.
Several long term studies have shown an excellent and sustained effect of SLT, but the fact remains that the efficacy of SLT diminishes in time [17-22]. Luckily, SLT has already proven to be repeatable and the effect of the repeat SLT to be equal to the original SLT [21,22,24].
But what do we tell our patients about the durability of their treatment? How many patients need to be retreated after which period of time? We set out to measure the need and the time it took to redo SLT after a first successful treatment.
One year after a successful SLT only 0.7% of our patients needed a redo. At 1.5 years 0.9%, 1% at 1.5 years, 5.6% at 2 years and 7.0% at 2.5 years. Kaplan-Meier survival analysis predicted a need to redo of 35.4% after 3 year, 40.4% after 3.5 years and 45.4% after 4.5 years.
Time to redo or time to failure had a mean of 31.13 ± 11.24 months in our study. This concurs with the results of Khouri et al. [21] who recorded a mean interval of 28.3 ± 12.7 months before repeat SLT was needed. Ayala et al.[35] recorded an average time to failure after SLT of 18 months. However, Ayala used 90° treatment of SLT and this is known to have less effect than a 360° treatment [31].
The need to redo and the time to redo were not correlated to any of the parameters we used like IOP at start, severity of glaucoma (VF, OCT, BCVA, cdr), age or kind of SLT treatment performed (primary, replacement, adjunct). This is in line with the fact that success of SLT in known to be independent of all parameters except IOP at start [32-34].
Shazly et al. [36] recorded a cumulative probability of success for patients to remain off medications for 2.5 years of approximately 75% in a treatment naïve group of patients. We had a cumulative probability of 81.0% after 2.5 years. This contrasts the low success rates of some of the older long term studies. Weinand et al.[17] recorded a success rate of SLT treatment of 60% after the first year, 53% after 2 years, 44% after 3 and 44% after 4 years. All of their patients showed advanced glaucoma uncontrollable by medication. In a comparable group of patients with uncontrolled IOP under maximal medication, Juzych et al. [18] recorded equally moderate success rates at 1, 3 and 5 years after SLT of 68%, 46% and 32%.
Keeping in mind that we eliminated the non-responders from our series and incorporated only the successful SLT treatments into further analysis, our patients had a one year survival rate of 99.0%, 93.5% after 2 years, 64.6% after 3 years and 54.6% after 4 years. These results are more in line with Gracner et al. [19] who had a success rate of 94% after 21 months, 85% after 24 months, 74% after 36 months and 68% after 48 months. The varying long term results probably reflect the populations that were treated in the different long term studies.
The current study is limited by its mixed population of patients with controlled and uncontrolled IOP and use of medication or not pre-SLT. This also allowed us to make a cross-section of a regular real-life population undergoing SLT treatment. It proved that differences in glaucomatous characteristics and kind of SLT had no influence on the outcome and the need for retreatment.
To date, there are limited peer reviewed publications on the time to redo SLT after first successful SLT treatment. Using a large population, long follow up and a prospective design allowed us to get a clearer view on the need to retreat.
We set out to have a general idea of how many patients could be expected to need a retreatment with SLT after a first successful SLT. In our population, the percentage of redo needed is 6.5% after 2 years, 35.4% after 3 years and 45.4% after 4 years. This allows us to give clear information to our patients about what to expect after SLT treatment.
Citation: Keyser M, Belder J, Ballet B, Mertens E (2020) Need and Time to Redo Selective Laser Trabeculoplasty in Glaucoma Patients. J Clin Exp Ophthalmol. 11:844. DOI: 10.35248/2155-9570.20.11.844
Received: 21-May-2020 Accepted: 04-Jun-2020 Published: 11-Jun-2020 , DOI: 10.35248/2155-9570.20.11.844
Copyright: © 2020 Keyser M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.