Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Short Communication - (2022)Volume 13, Issue 4
Neglected tropical diseases as been disease conditions that affect people particularly those living in the Tropical regions of the world, these disease entities affects many system of the body which therefore added to the burden of these disease. Some of these diseases affect the heart, blood vessels and other part of the cardiovascular system some presenting as life threatening conditions. Among these neglected tropical diseases that affect the cardiovascular system are schistosomiasis, African trypanosomiasis and Chagas disease which are the focus of this study. Schistosomiasis and African trypanosomiasis have been finding to be endemic in Africa while Chagas disease is found to be endemic in rural Americas communities. Some of these diseases especially schistosomiasis affects the heart causing the cardiomyocytes to fibros a condition called endomyocardial fibrosis while others causes some ischemic and inflammatory changes to the heart and other components of the cardiovascular system.
Neglected tropical diseases; Schistosomiasis; African trypanosomiasis; Chagas disease; Cardiovascular diseases
Neglected Tropical Diseases (NTDs) are infectious diseases that mostly affect the poorest people on the planet. They've been ignored for decades, first as part of a broader disrespect for the developing world, then more recently as a result of the increased focus on HIV/AIDS, tuberculosis, and malaria.
Chronic Non-Communicable Diseases (CNCDs) are becoming increasingly important in low- and middle-income countries with a moderate income (LMICs). These diseases are most prevalent in the tropics, although their preference for hot climates stems mostly from the fact that poverty is concentrated in remote rural communities, urban slums, and displaced populations near the equator. Rather than thinking of NTDs as tropical diseases, we should conceive of them as diseases affecting the “bottom billion.” The world's poorest one-sixth of the population, Start-up Murray and Lopez began dating to 1990s expected that death rates will double as a result of increase in NTDs in developing countries, bringing about rise in cardiovascular disease. Neglected Tropical Diseases (NTDs) and other poverty-related infections may account for a significant portion of cardiovascular disease in underserved groups.
On a worldwide scale, the contribution of poverty-related infections to heart disease can be evaluated by looking at WHO's Global Burden of Disease figures. “On a worldwide scale, the contribution of poverty-related infections to heart disease can be evaluated by looking at WHO's Global Burden of Disease figures [1].” Ischemic heart disease accounts for about half of the burden of cardiovascular illness, cerebrovascular disease for more than a third, and hypertensive and inflammatory causes, as well as rheumatic heart disease. NTDs and other neglected infections may account for a considerable portion of each of these cardiovascular diseases categories.
Among the neglected tropical diseases that have been indicated to have cardiovascular manifestation are schistosomiasis, African trypanosomiasis and chagas disease [2]. Schistosomiasis is a disease condition caused by parasitic infestation with schistosomiasis parasites which are Schistosoma masoni, Schistosoma Japenicum and schistosomiasis hematobium. African trypanosomiasis is also a parasitic infections caused by Trypanosoma gambiense and Trypanosoma rhodesiense while Chagas disease is also a form of trypanosomiasis which’s caused by Trypanosoma cruzi which is resident in the Americas.
As said earlier some neglected tropical disease has been indicated to affect the cardiovascular system and these article is considering the following schistosomiasis, African trypanosomiasis. The epidemiological studies are carefully considered below.
Schistosomiasis
Schistosomiasis is found to be prevalent in tropical and subtropical areas, particularly in poor communities without adequate access to clean and safe drinking water and proper sanitation. It is estimated that at least 90% of those requiring treatment for schistosomiasis live in Africa (Table 1).
| Types | Species | Geographical distribution |
|---|---|---|
| Intestinal schistosomiasis | Schistosoma mansoni | Africa, the middle east, the caribbean, brazil, venezuela and suriname |
| Schistosoma japonicum | China, indonesia, the philippines | |
| Schistosoma mekongi | Several districts of cambodia and the lao people’s democratic republic | |
| Schistosoma guineensis and related S. intercalatum | Rain forest areas of central Africa | |
| Urogenital schistosomiasis | Schistosoma haematobium | Africa, the middle east, corsica (France) |
Table 1: Adapted from world health organization, may 18th 2021 report [1].
Schistosoma hematobium a specie of schistosoma resident in the urinary tract but which can get to the systemic circulation to affect the cardiovascular system has been shown to be endemic in the sub-Saharan region of Africa and this includes Nigeria [2,3]. Approximately 200 million people in about 74 countries in the worlds are infected, with the risk of infection for at least 600 million people [4]. An estimation of about 120 million people suffers from severe consequences of the infection and an estimated annual mortality rate of about 20,000 globally [5]. On estimation about 30 million Nigerians needed to be treated for the disease annually [6]. In areas with high endemicity, the intensity of infection is found to be greatest in children within the age of 5 and 15 years [5].
African trypanosomiasis
As said earlier the African trypanosomiasis is of two major species, Trypanosoma gambiense and Trypanosoma Rhodesiense which commonly endemic to Africa and the epidemiological studies of each are considered below.
Trypanosoma gambiense
The study of the epidemiological prevalence of the disease caused by Trypanosoma gambiense in some African countries between the year 2000-2013 as reported by the World Health Organization was computed in the Table 2 below.
| Countries | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angola | 4,546 | 4,577 | 3,567 | 3,115 | 2,280 | 1,727 | 1,105 | 648 | 517 | 247 | 211 | 154 | 70 | 69 |
| Benin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. Faso | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cameroon | 27 | 14 | 32 | 33 | 17 | 3 | 15 | 7 | 13 | 24 | 16 | 15 | 7 | 6 |
| Chad | 153 | 138 | 715 | 222 | 483 | 190 | 276 | 97 | 196 | 510 | 232 | 276 | 197 | 193 |
| CAF | 988 | 718 | 572 | 539 | 738 | 666 | 460 | 654 | 1,194 | 1,054 | 395 | 132 | 381 | 62 |
| Congo | 111 | 894 | 1,005 | 717 | 873 | 389 | 300 | 189 | 182 | 87 | 87 | 61 | 39 | 20 |
| Cote d'Ivoire | 188 | 92 | 97 | 68 | 74 | 42 | 29 | 13 | 14 | 8 | 8 | 10 | 9 | 7 |
| DRC | 16,975 | 17,322 | 13,853 | 11,481 | 10,369 | 10,269 | 8,023 | 8,162 | 7,362 | 7,183 | 5,629 | 5,595 | 5,983 | 5,647 |
| Niger | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| Nigeria | 14 | 14 | 26 | 31 | 10 | 21 | 3 | 0 | 0 | 0 | 2 | 3 | 2 | 0 |
| Senegal | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| Sierra Leone | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| S. Sudan | 1,801 | 1,919 | 3,121 | 3,061 | 1,742 | 1,853 | 789 | 469 | 623 | 373 | 199 | 272 | 317 | 0 |
| Togo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Uganda | 940 | 310 | 604 | 517 | 378 | 311 | 290 | 120 | 198 | 99 | 101 | 44 | 20 | 0 |
| Total Reported | 25,865 | 26,117 | 23,836 | 19,963 | 17,130 | 15,644 | 11,382 | 10,473 | 10,388 | 9,685 | 6,978 | 6,637 | 7,106 | 0 |
Note: B.Faso=Burkina Faso, CAF=Central African Republic, DRC=Democratic Republic of Congo, E. Guinea=Equatorial Guinea, S. Sudan=South Sudan Source.
Table 2: Adapted from WHO 2003-2014 Report [7].
Trypanosoma rhodesiense
The study of the epidemiological prevalence of the disease caused by Trypanosoma rhodesiense in some African countries between the year 2000-2013 as reported by the World Health Organization was computed in the below Tables 2 and 3.
| Countries | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botswana | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| - | ||||||||||||||
| Burundi | - | - | - | - | - | - | - | - | - | - | - | _ | - | - |
| Ethiopia | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Kenya | 15 | 10 | 11 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 |
| Malawi | 35 | 38 | 43 | 70 | 48 | 41 | 58 | 50 | 49 | 39 | 29 | 23 | 18 | 35 |
| Mozambique | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Namibia | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Rwanda | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Swaziland | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Tanzania | 350 | 277 | 228 | 113 | 159 | 186 | 127 | 126 | 59 | 14 | 5 | 1 | 4 | 1 |
| Total Reported | 709 | 755 | 617 | 536 | 552 | 710 | 453 | 305 | 259 | 190 | 156 | 113 | 110 | 86 |
Table 3: World health organization 2003-2013 report [7].
The figures below show the geographical distribution of the human African trypanosomiasis (Figure 1).
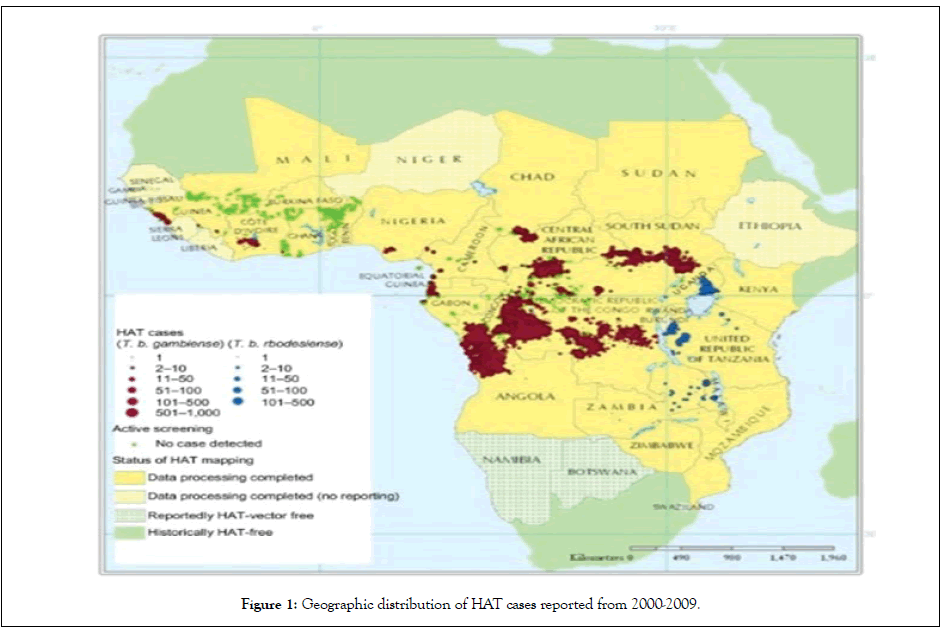
Figure 1: Geographic distribution of HAT cases reported from 2000-2009.
Chagas disease
This disease is caused by a specie of trypanosoma named Trypanosoma cruzi, this organism is not commonly found in Africa but it’s endemic in some other part of the world especially the rural region of Americas. It’s found to be endemic in about 21 America's countries and affecting about 6 million people [7]. It's an annual incidence of about 30,000 new cases, 12,000 deaths and 8,000 congenital infections of newborns in the Americas [7].
The cardiovascular manifestation varies in each of these diseases and this section is critically reviewing each.
Schistosomiasis
A study have shown increased incidence of Pulmonary Artery Hypertension (PAH) leading to cor pulmonale in patients with Schistosoma masoni and Schistosoma Japonicum infections [8]. Infact, a study shows about 4.6% increase incidence of pulmonary artery hypertension in patient with schitosomiasis [9].
A case of schistosomal pericarditis in a 16 years old African female was reported by Horst, et al. [10]. This was as a result of oval of Schistosoma hematobium forming granulation leading to scarring and fibrosis of the pericardium.
The pathophysiology of the pulmonary artery hypertension has been linked to back systemic hypertension resulting from portal hypertension due to obstruction of portal circulation by ova of Schistosoma masoni [11]. Lapa et al. calculated an estimate of 200 million people worldwide are infected with any Schistosoma species, of whom 4%-8% develop hepatosplenic disease, and greater than 270,000 will go on to develop pulmonary artery hypertension [12]. The endomycardial fibrosis resulting from the pulmonary artery hypertension is associated with pericarditis, arrhythmias, and mural thrombi [13]. Endomycardial fibrosis is rampant in the tropics and it’s the fourth leading cause of heart disease in Nigeria [14].
African trypanosomiasis
African trypanosomiasis can be associated with myocarditis and pericarditis, especially in the acute stages of the illness when the trypamastigote stages of the parasite spread through the blood and lymphatics to cause [15].There is increasing incidence of cardiovascular involvement in African trypanosomiasis infection [15].
Most of the cardiovascular manifestations of African trypanosomiasis are seen as result of lymphohistocytic infiltrations leading to edema in the pericardium, myocardi and endocardium [16].
Chagas disease
Some cardiological manifestations have been associated with Chagas disease, they’re refer to as chagasic cardiomyopathies including heart failures, arrhythmias mural thrombi leading to pulmonary and systemic emboli, and also sudden death [17,18]. The chronic heart failure has been thought to be due to the persistent presence of the amastigote of the trypanosome in the heart leading to a pathological cascade of tissue destruction, myocarditis, fibrosis and ventricular dilation [19]. The arrhythmias can be attributed to the fibrosis, the arrhythmia predisposes to emboli formation especially cardiac mural emboli causing increase incidence of cerebrovascular accident [18,20].
The neglected tropical disease especially the schitosomiasis, African trypanosomiasis and Chagas disease have been on the global scale a latent cause of cardiovascular disease which need to be given attention to most especially in the tropics where these diseases are endemic. Endo-myocardial fibrosis has been reported fourth leading cause of cardiomyopathy in Nigeria the biggest African nation. Consequently, about 2.3 million individuals at some random time might be impacted by Chagas cardiomyopathy, which can introduce either as ischemic or provocative coronary illness or with blended highlights of both.
The authors acknowledge the effort of the editor-in-chief and members of Ladoke Akintola University Medical (LAUMED) Journal Club for their great efforts towards this article.
We acknowledge Dr. M Akinlade, FWACP, FRCP a consultant cardiologist who supervised the processes that resulted in this article.
Authors declared no conflict of interest.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar][PubMed].
Citation: Ojo R, Alare K, Adekanye I, Odedel T, Oladokun O, Akindele Z (2022) Cardiovascular Diseases: A Systematic Review of Cardiovascular Manifestations of Schistosomiasis, African Trypanosomiasis and Chagas Disease. J Clin Exp Cardiolog. 13:721.
Received: 25-Apr-2022, Manuscript No. JCEC-22-17131; Editor assigned: 28-Apr-2022, Pre QC No. JCEC-22-17131 (PQ); Reviewed: 16-May-2022, QC No. JCEC-22-17131; Revised: 23-May-2022, Manuscript No. JCEC-22-17131 (R); Published: 30-May-2022 , DOI: 10.35248/2155-9880.22.13.721
Copyright: © 2022 Ojo R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.