
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Mini Review - (2021)Volume 12, Issue 1
Neutrophil; Macrophage; Hypoxia; Inflammation; Infection
AMP: Antimicrobial Peptides; BMDM: Murine Bone Marrow- Derived Macrophages; HIF: Hipoxia Inducible Factor; HRE: Hypoxic Response Elements; HCLO: Hypochlorous Acid; IL-1: Interleukin-1; iNOS: Inducible Nitric Oxide Synthase; mRNA: Messenger Ribonucleic Acid; LPS: Lipopolysaccharide; MDM: Monocyte-Derived Macrophages; MMP: Metalloproteinase; Mtb: Mycobacterium Tuberculosis; NETs: Neutrophil Extracellular Traps; NFkB: Nuclear Factor Kappa B; NO: Nitric Oxide; PAF: Platelet-Activating Factor; PHD: Prolyl Hydroxylase; ROS: Reactive Oxygen Species; TLR: Toll-Like Receptor; TNF-α: Tumor Necrosis Factor Alpha; VEGF: Vascular Endothelial Growth factor; vHL: Von Hippel–Lindau Tumor-Suppressor Protein
The elimination of pathogens depends initially on the innate immune response that pre-exists in all individuals. Neutrophils and macrophages are the main effectors in innate immunity because they can detect, phagocyte, and eliminate pathogens without the help of an adaptive immune response. Neutrophils and macrophages reflect their ability to function in hypoxia when they migrate to healthy tissues and are exposed to an oxygen tension that is generally 20–70 mmHg or to tissues with inflammation or necrosis where oxygen tension drops below 20 mmHg [1-3].
The adaptive response of neutrophils and macrophages to hypoxia is regulated by the action of hypoxia inducible factor 1 (HIF-1). HIF-1 is a heterodimer whose expression is regulated by the presence of oxygen in a tissue. Prolyl hydroxylases (PHD) regulate the stability of the α subunit (HIF-1α) by adding a hydroxyl group to HIF which then interacts with the von Hippel–Lindau tumorsuppressor protein (vHL) and HIF is ubiquitinated to be eliminated in the proteasome. In hypoxia the action of the prolyl hydroxylase is inhibited, and HIF-1α accumulates in the cytoplasm and translocates into the nucleus, where it binds to HIF-1β and they activate the Hypoxic Response Elements (HREs) in the nucleus, increasing the transcription of genes of glycolysis, proinflammatory cytokines, vascular endothelial growth factor (VEGF) and inducible nitric oxide synthase (iNOS) implicated in the control of metabolism, angiogenesis, and elimination of pathogens [4,5].
Oxygen is vital for life and its availability impacts on various physiological and pathophysiological processes across the human body [6]. Organs and tissues have different oxygen tensions and this differences are of central importance for normal organ function, for example, in lungs, blood vessels, bone marrow, cartilage, liver, and kidney [7,8]. Hypoxia is present in acute and chronic diseases and, depending on the magnitude and duration, can be either beneficial or harmful for tissue recovery [9]. Oxygen tension varies across the human body, for example: 150 mmHg (lung apices), 100 mmHg (alveoli and arterial blood), to <20 mmHg (bone marrow) [10]. In this review, we will focus on how neutrophils and macrophages respond to hypoxia in the context of inflammation and infection (Figure 1) [11].
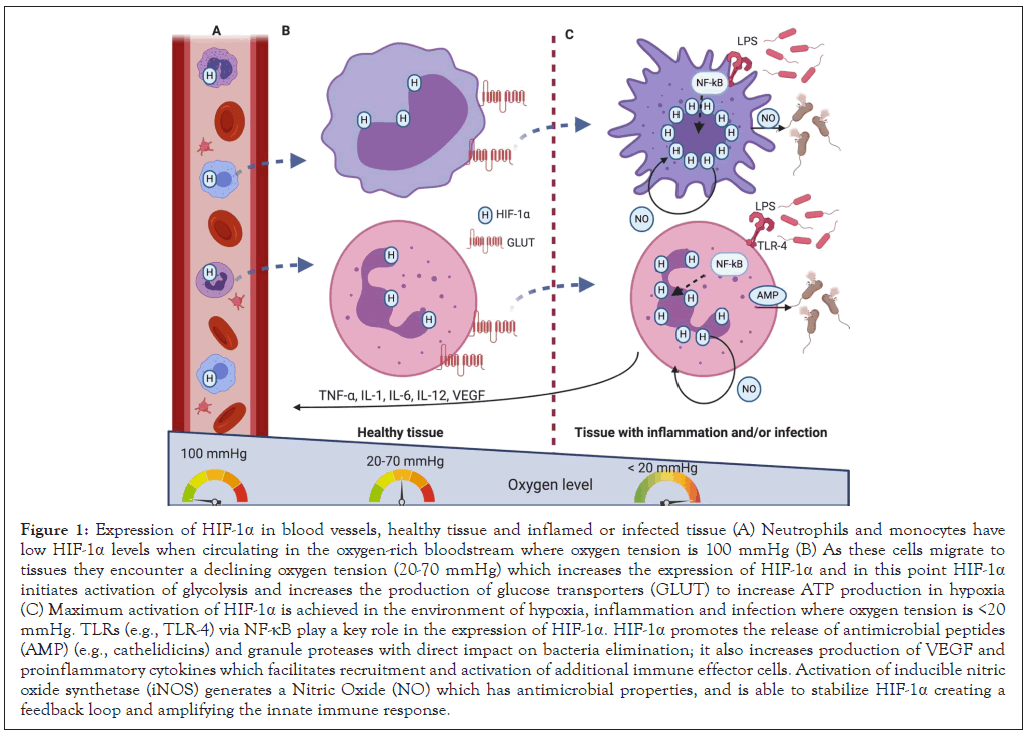
Figure 1: Expression of HIF-1α in blood vessels, healthy tissue and inflamed or infected tissue (A) Neutrophils and monocytes have low HIF-1α levels when circulating in the oxygen-rich bloodstream where oxygen tension is 100 mmHg (B) As these cells migrate to tissues they encounter a declining oxygen tension (20-70 mmHg) which increases the expression of HIF-1α and in this point HIF-1α initiates activation of glycolysis and increases the production of glucose transporters (GLUT) to increase ATP production in hypoxia (C) Maximum activation of HIF-1α is achieved in the environment of hypoxia, inflammation and infection where oxygen tension is <20 mmHg. TLRs (e.g., TLR-4) via NF-κB play a key role in the expression of HIF-1α. HIF-1α promotes the release of antimicrobial peptides (AMP) (e.g., cathelidicins) and granule proteases with direct impact on bacteria elimination; it also increases production of VEGF and proinflammatory cytokines which facilitates recruitment and activation of additional immune effector cells. Activation of inducible nitric oxide synthetase (iNOS) generates a Nitric Oxide (NO) which has antimicrobial properties, and is able to stabilize HIF-1α creating a feedback loop and amplifying the innate immune response.
Inflammation is a complex and ordered sequence of events that includes redness, heat, swelling, and pain. Inflammation can be triggered by infection, chemicals, radiation, and mechanical force. Neutrophils and macrophages are very important in inflammation because they are usually found in high numbers and with a proinflammatory profile in inflamed tissues. Neutrophils and macrophages not only engulf and kill microorganisms, but also promote the activation of lymphocytes, fibroblasts, and endothelial cells which are important for pathogen control and tissue/organ reconstitution [12,13]. Inflammation is intimately linked to oxygen metabolism [14]. It is important to note that, while in inflammation increased blood flow suggests an increase in oxygen delivery, inflamed tissues are usually hypoxic [15-17]. This could be happening due to higher interstitial pressure (swelling) and increased oxygen consumption of cells in their fight to survive the harsh conditions of inflamed tissues. Neutrophil respiratory burst has been thought to contribute to inflammation-associated hypoxia before, Campbell et al. presented evidence for a functional role of neutrophils in oxygen consumption during colitis and the induction of a hypoxic response in intestinal epithelial cells [18]. Furthermore, mice with a defective respiratory burst (Nox2−/− mice, a model system for chronic granulomatous disease) displayed severe impairment of inflammatory resolution in the gut, supporting the notion that hypoxia and hypoxia-induced responses are functionally relevant for various aspects of the pathogenesis of inflammation [18]. The intimate link between hypoxia and inflammation is also demonstrated by the observation that hypoxia by itself can induce an inflammatory response [14]. Mice that were exposed to 5% O2 for 60 minutes had an increased protein expression of IL-6, TNF-α, and IL-1 in both serum and isolated macrophages [19]. In healthy human volunteers that stayed 3 nights at high altitudes there was an increase in serum levels of proinflammatory proteins [20].
Macrophages response to hypoxia in vitro is complex and determined by the source and phenotype of the macrophage as well as the culture conditions. Hypoxia affects the expression of cell surface markers, viability, phagocytosis, metabolic activity, and cytokine release of macrophages [21]. The clinical relevance of hypoxia induced inflammation is demonstrated in lung and kidney transplants where an ischemia-associated inflammatory reaction increases the risk of transplant failure and graft rejection [22,23]. All of the above shows us the possibility of a vicious circle where hypoxia and inflammation work together and mutually boost each other [24]. With all of this information it is reasonable to assume that the molecular mechanisms that induce the hypoxiainflammation vicious circle represent possible therapeutic targets for the treatment of chronic, non-resolving inflammation thus helping prevent organ failure [11,25,26].
The reason that hypoxia is present during an infection associated inflammation is multifactorial, and involves an augmented oxygen consumption in order to fulfill the requirements of inflamed resident cells like macrophages, infiltrating inflammatory cells like neutrophils and monocytes and also multiplying pathogens [18,27,28]. A chronic inflamed tissue associated with a chronic infection leads to decreased blood supply resulting in the combination of vascular pathology and microthrombosis [28]. The combination of an increased oxygen consumption and decreased supply contributes to the presence of hypoxia during an infection. Tissue hypoxia has been demonstrated in vivo during a range of infections. Stabilization of HIF-1 in host cells has been reported during bacterial infections with several pathogens [29], and, in most cases, HIF-1 stabilization is associated with increased bacterial killing, but it also depends whether the infection is local or systemic [5,30].
Bacterial infection can lead to an increased HIF activation through hypoxia-independent pathways, including the upregulation of HIF- 1α mRNA levels by NF-κB, which is activated in response to the activation of toll-like receptor (TLR-4) by bacterial antigens (LPS) [31].
Lipopolysaccharide (LPS) can increase succinate accumulation which promotes PHD inhibition with subsequent HIF stabilization [32]. Although the activation of HIF-1 promotes infection control, it does not universally increase cellular capacity to clear invading pathogens. In some cases, pathogens can use HIF-1 to increase their pathognomic potential. For example, Bartonella henselae via HIF- 1 increases vascular endothelial growth factor (VEGF) expression in cells leading to vascular proliferations [33-35]. It also depends whether the infection is local or systemic [5,29,34] This means that using HIF-1 as a therapeutic target requires knowing the pathogen that is causing the infection and whether this infection is local or systemic.
Neutrophils are the most abundant cell of the innate immune system and they are recruited to wounds and infections during the early phase of the disease. Neutrophils are attracted to inflammatory tissues via IL-8, C5a, N-formylated peptides, platelet-activating factor (PAF), and leukotriene B4 [36]. After detecting bacteria or inflammation mediators, neutrophils engulf pathogens followed by the activation of the electron transport chain (NADPH oxidase) which releases electrons across the membrane to molecular oxygen for the generation of hypochlorous acid (HClO) and reactive oxygen species (ROS) leading to the elimination of the pathogen [37]. This process is called “respiratory burst” and it consumes an elevated quantity of oxygen [38]. The respiratory burst is an essential antimicrobial pathway of neutrophils. Neutrophils can also kill pathogens via release of antimicrobial peptides (AMP), inflammatory cytokines like TNF-α, IL-1, IL-6 and in some cases they generate extracellular traps [39]. The presence of neutrophils at sites of inflammation or infection results in oxygen consumption, this phenomenon is called “inflammatory hypoxia” [11,39].
Inflamed tissue like in type 2 diabetes becomes extremely hypoxic when there is an increased oxygen demand and the availability decreases due to swelling, trauma, or thrombosis [14-17]. Neutrophils have a half-life of approximately 6 to 8 hours, but in the presence of HIF neutrophils increase their survival within inflammatory tissues, giving them the time to properly eliminate a pathogen [36]. The inhibition of neutrophil apoptosis and increased survival associated with hypoxia was demonstrated to be NF-κB-dependent, showing that NF-κB is a regulator of the hypoxic response in neutrophils [40]. In the presence of hypoxia, HIF facilitates neutrophil binding to the epithelium via the increased expression of β2 integrin [41]. In terms of ATP generation, neutrophils rely on high rates of glycolysis in which HIF-1α plays a key role by regulating the expression of key glycolytic enzymes [42]. HIF-1 also increases neutrophil expression of antimicrobial peptides (AMP), this is suggested by experiments in which HIF-1αdeficiency increases the susceptibility of local and systemic bacterial infections [5,42].
The role of another HIF subunit (HIF-2α) during neutrophilic inflammation is less known. Deficiency of HIF-2α in murine neutrophils did not affect chemotaxis, phagocytosis, or respiratory burst, but it did increase apoptosis resulting in a reduced neutrophilic inflammation [43]. Neutrophils with an increased expression of HIF-2α had lower apoptosis rates, suggesting a key role of HIF-2α in the resolution of inflammation [11].
In the presence of HIF neutrophils increase their survival extending their half-life beyond 8 hours [44]. Neutrophils oxidative burst consumes a lot of oxygen, generating a hypoxic environment and this could be happening during active TB when they infiltrate the lung. Neutrophils prolonged survival in response to hypoxia, infection and inflammation is related to a sustained expression of PHD3 [45]. This is important because in the lungs of Mtb infected mice there are an increased expression of PHD3 [46]. HIF-2α is highly expressed in neutrophils, whereas HIF-1α is highly expressed in macrophages. HIF-2α deficiency increases neutrophil apoptosis [43]. Neutrophils contribute in the innate immune response [47,48] and granuloma formation [49-51] in the early phase of Mtb infection. As the neutrophil migrates through the granuloma, oxygen tension decreases and there is an increased neutrophil degranulation and tissue damage [45]. In an early phase of Mm infection HIF-1α increases NO production in neutrophils and increases the elimination of the bacteria [52]. Human neutrophils stimulated with Mtb and hypoxia increase the secretion of MMP- 8, MMP-9 and neutrophil elastase, which are involved in matrix destruction. Hypoxia inhibits NETs formation, apoptosis and necrosis after exposure to Mtb [44,53].
Macrophages are phagocytic cells involved in many pathological processes like inflammation, wound healing, atherosclerosis, and tumors. All of these processes are characterized by hypoxia [54]. Hypoxia is present in inflamed tissues and macrophages are able to adapt to this hostile microenvironment. This is possible by the stabilization of HIF in macrophages which is found at various stages of activation and polarization [55,56]. Inhibition of HIF affects macrophage functions such as aggregation, migration, and invasion [11,36,42,57].
In HIF-1α-deficient macrophages low levels of intracellular ATP were detected, confirming the importance of HIF-1α for energy generation via glycolysis in myeloid cells [58]. Hypoxia is present in sterile inflammation and infection [57]. Macrophages are part of the first line of defense against pathogens and it has been hypothesized that these cells must be able to function in hypoxic areas. It was shown via gene ablation in mice, that the expression of HIF-1α in macrophages is extremely important for the elimination of pathogens [5,42]. The antimicrobial effect of HIF-1α is present in hypoxia, but also under ambient air. It was observed that under normoxia and bacterial infection, the stabilization of HIF- 1α increases in macrophages [5]. In terms of bacterial antigens, lipopolysaccharide (LPS) is capable of inducing mRNA expression and HIF-1α protein accumulation in murine macrophages and human monocytes under normoxia [59,60]. Because of this, it was suggested that toll-like receptor 4 (TLR-4) could play a key role in HIF-1α activation. Later on it was discovered that HIF-1α and TLRs interact with each other in a feedback loop on many different levels [61]. HIF-1α regulates the expression and signal transduction of several TLRs (e.g., TLR-2, -3, -4, -6, -7/8 and -9) [62-67]. It is important to remember that NF-αB is crucial for TLR signaling and that it is induced by LPS [68]. Thus, the activation of HIF-1α by LPS is dependent on NF-αB in human monocytes and murine macrophages [59,69]. In summary, the activation of TLR by HIF- 1α shows the importance of HIF-1α in the control of pathogens. Compared to HIF-1α, there is little information about HIF-2α expression in macrophages. Under hypoxia HIF-2α expression increases in several myeloid cell types, for example, human monocyte-derived macrophages (MDM) and murine bone marrowderived macrophages (BMDM) [70,71]. Functional inactivation of HIF-2α in macrophages resulted in a decreased response to hypoxia and to proinflammatory stimulation with LPS plus interferon-γ [55,70]. HIF-1α and HIF-2α function in macrophages is not always redundant; they have specific regulation of selected factors [72]. For example, deletion of HIF-2α in macrophages does not impact the expression of the inducible nitric oxide synthase (iNOS) and the vascular endothelial growth factor (VEGF) which are target genes of HIF-1α [71,73]. In contrast, HIF-2α activates soluble VEGF receptor-1, while HIF-1α has no effect [11,74].
In an infection by Mycobacterium tuberculosis (Mtb) there are several host factors that contribute to a higher expression of HIF-1α. One factor is nitric oxide (NO) which is induced by the action of an inducible nitric oxide synthase (iNOS) in infected murine macrophages. iNOS expression produces a redistribution of intracellular oxygen and inhibition of PHDs forming a positive feedback loop that leads to an increased expression of HIF-1α which increases macrophage activation. The other factor is NF- κB, its expression is increased in alveolar macrophages of mice and rabbits during Mtb infection, as well as in the granulomas of patients with pulmonary tuberculosis where hypoxia is present. NF- κB translocates into the nucleus and activates the transcription of HIF-1α and other target genes which in turn control Mtb growth [46,73] (Figure 1).
As mentioned before, using HIF-1 as a therapeutic target requires knowing the pathogen that is causing the infection and whether this infection is local or systemic. The comorbidity of pulmonary tuberculosis and type 2 diabetes is a great example of the 3 things mentioned in this article, infection, inflammation and hypoxia, all at the same time with the stimulation of bacterial antigens and advanced glycation end products derived from chronic hyperglycemia (Figure 2). In this type of situation and with the increasing antibiotic resistance from Mtb and many bacteria, the modulation of HIF-1α and HIF-2α could be a great adjuvant therapy alongside antibiotics, but there is still a long way to go to understand the function of HIF-1α and HIF-2α in this types of diseases, like the comorbidity of pulmonary tuberculosis and type 2 diabetes.
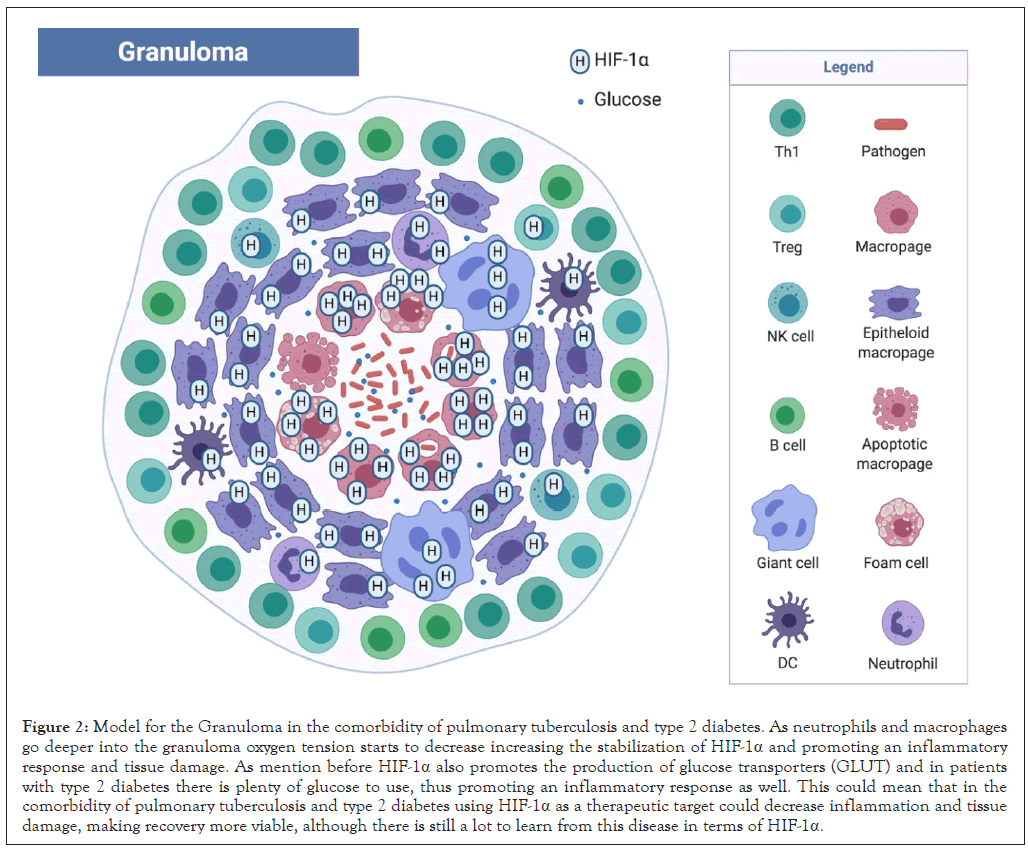
Figure 2: Model for the Granuloma in the comorbidity of pulmonary tuberculosis and type 2 diabetes. As neutrophils and macrophages go deeper into the granuloma oxygen tension starts to decrease increasing the stabilization of HIF-1α and promoting an inflammatory response and tissue damage. As mention before HIF-1α also promotes the production of glucose transporters (GLUT) and in patients with type 2 diabetes there is plenty of glucose to use, thus promoting an inflammatory response as well. This could mean that in the comorbidity of pulmonary tuberculosis and type 2 diabetes using HIF-1α as a therapeutic target could decrease inflammation and tissue damage, making recovery more viable, although there is still a lot to learn from this disease in terms of HIF-1α.
Hypoxia, inflammation and infection are 3 things that create a hostile environment for neutrophils and macrophages, this is the main cells in innate immunity and they have adapted to function in these conditions. HIFs are absolutely essential for proper cell function under hypoxic conditions. Although there is a lot of literature on the role of HIF-1α, the role of HIF-2α remains elusive, and whether there are more HIF proteins that could affect the function of neutrophils and macrophages.
Another key question is how effective the modulation of HIF will prove to be in the therapy of acute and chronic inflammation with hypoxia and infection and what kind of side effects will appear. Will the modulation of HIF be effective as an adjuvant therapy alongside antibiotics? These questions, among others, will have to be studied in order to find a successful therapy in patients with chronic inflammatory diseases which are susceptible to chronic infections, that lead to chronic hypoxia, tissue damage and organ failure.
All the figures were created with Biorender.com.
The authors declare that there is no conflict of interests regarding the publication of this paper.
Citation: Muñiz-Buenrostro A, Carmona MCS, Meléndez RCC, Mendoza AYA (2021) Neutrophil and Macrophage Response to Hypoxia in the Context of Inflammatory and Infectious Diseases. J Clin Cell Immunol. 12:610.
Received: 21-Jan-2021 Accepted: 04-Feb-2021 Published: 11-Feb-2021 , DOI: 10.35248/2155-9899.21.12.610
Copyright: © 2021 Muñiz-Buenrostro A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.