
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)Volume 11, Issue 6
Purpose: The adaptation of monitoring activities to the pandemic situation by COVID-19 has been reviewed in this article. The opinion of the personnel involved in the different stages of the clinical trial has been analyzed and compared with the exceptional measures proposed by the official authorities for the follow up of clinical research.
Method: During the months of July and August 2020, a cross-sectional descriptive study was started up using an online questionnaire with 53 items to evaluate the opinion of study coordinators, clinical research associated and project managers from public research institutes and private companies.
Results: The survey was completed by 107 clinical research professionals from the public and private sectors. It shows qualitative data related to their demographic information and significant differences in terms of public and private enterprise to adapt to the pandemic in terms of clinical trial follow-up visits, medication management, communication with the center and accesses to data verification.
Conclusion: When analyzing the data after a health emergency, we ask ourselves whether the measures applied are effective in maintaining the activity of the clinical trial and whether they comply with current legislation to guarantee patient confidentiality and safety. Recommendations are established for situations of this type, both for the clinical research professionals and for the patients involved in the clinical trial.
Pandemic; Clinical research; COVID-19; Clinical trials
The monitoring of a clinical trial is an essential activity to guarantee the rights, safety and well-being of the participating subjects, as well as the solidity and reliability of the data obtained. Its application is necessary for compliance with good clinical practice and current legislation on Clinical Trials (CT).
During the state of alert in SPAIN, the Spanish Agency of Medicines and Health Products (AEMPS) and the European Medicines Agency (EMA) published a document of exceptional measures applicable to the CT to manage the problems derived from the COVID-19, to guarantee the activity of the trials and the traceability of the implemented actions, as well as with the action plans for the notifications to the Clinical Research Ethics Committees (CEIm) and AEMPS.
On-site monitoring at sites has been partially or fully restricted during the pandemic, and monitoring plans have been adapted to continue providing the necessary support to the site and the study sponsor. According to this situation the research team has modified its clinical practice to comply with health recommendations, maintaining the quality of care and research activity.
Objectives
• To identify new strategies for monitoring activities that emerged during the COVID-19 pandemic.
• To verify the follow-ups conducted by the personnel involved in the development of the clinical trial.
Study design
A cross-sectional study was conducted through a survey aimed at professionals working in the pharmaceutical industry and public health foundations during the months of July, August and September 2020 (4 months after the declaration of the state of alarm in Spain).
Settings
The questionnaires were developed by professionals who are currently working on research projects, observational studies and clinical trials. Their experience in formulating the questions is based on the coordination of studies, monitoring and management of projects, which is why it is highly adapted to clinical research and to Spanish health centers.
The survey consisted of 4 parts with 53 open and closed questions. The first block consists of 18 items about the sociodemographic and labor characteristics of the respondents and information regarding monitoring activities. A second part contained information on COVID and non COVID studies conducted during the pandemic composed of 12 items of closed questions. The third block refers to monitoring adaptation: types and objectives of contacts made and medication management. This part is evaluated through 15 open and closed questions.
The conclusions of the study are obtained in the last part of the survey, which consists of 8 questions of categorical type: 7 questions of positive and negative aspects about the monitoring activity carried out during the pandemic and also 1 open question that allows each participant to include personal opinions
The survey was carried out using the Google Form tool enabled during the months of July, August and September 2020.
Participants
The personnel who answered the survey work in public and/or private companies carrying out activities focused on the coordination, monitoring and management of clinical trials.
The survey was published on a social network and sent by email to the group of professionals belonging to the RED ESPAÑOLA DE INVESTIGACIÓN CLÍNICA (SCReN), so people who meet the following criteria were included:
• Professionals working in private or public companies with clinical trials coordination, supervision or management functions.
• Professionals over 20 years of age.
• Professionals with experience between 1 and 10 years in public or private companies.
• Professionals working during the state of alert in SPAIN from March 15, 2020 to June 1, 2020.
• Professionals who do not give up the data obtained in the survey.
Variables
• Number of COVID studies monitored versus non-COVID studies, and mode of visit.
• Number of initiation, monitoring and final visits made in the COVID versus non-COVID studies and types of access to source documentation.
• Analysis of treatment adherence by the monitor and study coordinator.
• Evaluation of the positive and negative effects of remote monitoring modalities.
Data sources/measurement
The sample is made up of both men and women, with ages between 20 and 45. They all have between 1 to 10 years of experience in clinical research and work in private or public companies with functions of coordination, supervision or management of clinical trials.
The sample size was estimated based on a specific time period of three months (July-September 2020), which was settled to receive the information from the participants.
Study size
The study is classified as a cross-sectional study conducted at a given time, from July to September, no pre-set sample size was established.
Following the request to the participants of the authorization to use their data in accordance with the REGULATION (EU) 2016/679 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 April 2016 on the protection of individuals with regard to the processing of personal data and on the free movement of such data and repealing Directive 95/46/EC (General Data Protection Regulation), the survey was published on a social network and sent by e-mail to the group of professionals belonging to the Spanish Clinical Research Network (SCReN).
In this process the anonymity of the participants is preserved and no exclusion criteria are described.
Quantitative variables
• Number of COVID studies monitored versus non-COVID studies, and mode of visit.
• Number of initiation, monitoring and final visits made in the COVID versus non-COVID studies and types of access to source documentation.
Statistical methods
To estimate the qualitative data from the survey, descriptive frequency analysis was performed: mean and standard deviation.
The chi-square test was used to compare proportions. Differences were considered significant when the p<0.05. The IBM SPSS Statistics 20 statistical program was used.
Participants
We obtained data from 110 professionals; however, we finally analysed the answers from 107 of them, as there were 3 professionals who did not consent to the use of their data.
Descriptive data
Demographic characteristics: The sample was made up of both men (19.1%) and women (80.9%). The subjects were divided for range of age in seven categories: 20-25, 26-30, 31-35, 36-40, 41-45 and >45.
The most frequent range of age in our study was 31-35 (with a total of 26.4% participants in that group: 22.2% of them worked in public entities and 31.9% in private companies).
The work experience in clinical trials of the respondents was analyzed, with a mean (x) of 5.43, typical dev. 2.966 and variance of 8.797.
When asking for the localization of the participants, we found out that most of them worked in large communities such as Madrid (44.5%), Barcelona (18.2%) followed by Andalucia (9.1%). We would like to note that no response was obtained from personnel working in Asturias, Balearic Islands, Castilla La Mancha, Extremadura, La Rioja, Navarra and the two autonomous cities Ceuta and Melilla (this fact is due to the displacement of the monitors from the central sites to that cities).
The professional profiles of the subjects were as follows: 43.6% of the respondents worked as monitors, 8.2% as project managers and 30.9% as study coordinators. The rest of the percentage of professionals, had mixed profiles between monitor, manager and coordinator (17.3%), and a high number of them, (95.9%) developed their activity in public companies (Table 1).
| Demographic characteristics of survey participants | |
|---|---|
| Gender | Female/Male |
| Age | 20-25 |
| 26-30 | |
| 31-35 | |
| 36-40 | |
| 41-45 | |
| >45 | |
| Affiliation | Public/Private |
| Work experience | 1-10 years |
| Autonomous community | Andalucía, Aragón, Asturias, Baleares, Canarias, Cantabria, Castilla-La Mancha, Castilla y León, Cataluña, Ceuta, Comunidad Valenciana, Extremadura, Galicia, La Rioja, Madrid, Melilla, Murcia, Navarra, País Vasco. |
| Professional profile | Monitor, Project manager and study Coordinator. |
Table 1: The characteristics of the population are as follows.
Outcome data
General information: We compared general monitoring information from the pre-pandemic situation with that available from the post-pandemic appearance.
We also divided the results from the post-pandemic stage in two types: one with information related to COVID-19 studies and another one for non-COVID-19 studies.
In this study, 70% of the respondents reported to have made remote visits during that period of time and, of them, a total of 55.5% claimed that they had only carried out 25% of the studies commissioned remotely before the pandemic.
We divided, as previously mentioned, the kind of activity in COVID-19 related-studies or non-COVID-19 studies. The mean of COVID-19 studies started was 8.94 (dev=11.77, variance=138.43) compared to 1.05 of non-COVID-19 studies (dev=1.57 and variance=2.47).
We wanted to find out the percentage of activity that had been modified due to the pandemic. A total of 57.3% subjects reported that their projects had not been paralyzed, in contrast with 18.2% of subjects who informed that all of their projects were paralyzed. When comparing data from the public entities and private companies, there were no statistically significant differences between groups (p=0.705).
In order to obtain more specific information, we classified the remote visits performed into the 3 phases of a clinical trial: initiation visit, monitoring visit and end-of-trial visit. In the survey, we asked the subjects to give an approximate answer about the number of remote visits performed: less than 50%, between 50%-75% and more than 75%, and we used this classification into three categories.
When analyzing the results, we can observe that no end-of-trial visits were performed in remote (both in public and private sectors); a higher number of subjects (n=95, 86.4%) informed that they had performed a little number of initiation remote visits: less than 25% of the total visits. A total of 82.5% corresponded to public centers compared to 91.5% in private companies, with no significant differences observed between both sectors (p=0.399).
In global, the monitoring activity needed to guarantee the continuity of the clinical trials, was reported to had been done remotely in more than 75% of the cases (public centers: 47.6%, private companies: 68.1%; with no significant differences observed between both sectors, p=0.072.
Adapting new strategies: Types and objectives
To carry out remote monitoring, some aspects need to be defined. One point is to establish which communication channels to use, as: e-mail, video call/teleconference or telephone call, with the possibility of using the combination of them all.
In our study, the majority contact was found out to be the combination of telephone call and email (57.3%), followed by contacts by e-mail (24.5%), and in a few percentages of cases (0.9%) a combination of video calls with email/phone contact was employed. No subject reported to use uniquely the video call as contact tool.
There is a significant difference between the communication channels used in public versus private sector: In the public centers, the e-mail was the main option, while the employees from the private companies referred to use a combination of email and phone calls (p=0.002)
Other important aspect to carry out the monitoring activity according to current legislation is the properly review of the monitoring documents: the monitor must have access to the clinical history to be able to review the data. When comparing the answers from the participants of our study, we found out significant differences between private and public companies: a 63.5% of subjects working in public companies reported to have had access to source documents, compared to a 19.1% of workers in private companies (p=0.00). The access modalities can be seen in Figure 1.
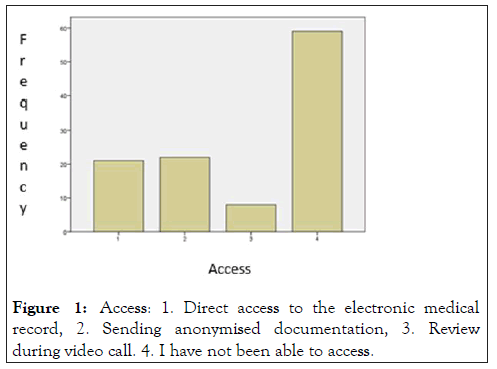
Figure 1: Access: 1. Direct access to the electronic medical record, 2. Sending anonymised documentation, 3. Review during video call. 4. I have not been able to access.
The main reasons reported for not having access to the medical history were those that follow in Table 2.
| Reasons |
|---|
| Impossibility of anonymizing the documentation by the research team |
| Not allowed by AEMPS. |
| Not allowed to data confidentiality |
| Visits have been postponed until the end of the state of alarm. |
| Not applicable due to access to electronic records |
Table 2: Reasons for not accessing medical records.
Another fact we analyzed was the change in the monitoring visit modality, with a change in the face-to-face visits to remote visits in 30% of the cases and with a total of 66.4% of the visits performed remote (with no significant differences observed between the public and the private entities, p=0.594). We also asked about study medication issues. The 90.9% of the subjects informed that they did not work with home delivery of medication before the COVID-19 pandemic. However, after the pandemic appearance, these data have undergone a radical change: 54.5% of the survey participants reported that they had begun to send medication. No significant differences between public and private centers in this aspect were found (p=0.367). Both entities adapted to the new circumstances due to the need to maintain patients with clinical trials treatments [1,2].
In order to check adherence to treatment, different ways were employed: information reported by phone call, photo or journal delivery, some survey participants referred to check it through a family member designed to deliver the medication to the site.
However, there were also cases were the checking of adherence was postponed until face-to-face visits were restored. And additionally, in some cases this item was not applicable: those studies in which medication is from hospital administration.
Key results
In this study, we aimed to evaluate the status of monitoring activities during the pandemic by SARS-CoV-2 declared by the WHO [1]. We specifically studied the months of March to June 2020, coinciding with the time period in which the Spanish government published the Real Decreto 926/2020, of October 25, which refers to the state of alarm to contain the spread of infections caused by SARS-CoV-2 [2].
The monitoring of clinical trials was compromised throughout the time period described above. There was a need for the development and implementation of new strategies. The official reference medical agencies (such as the AEMPS and the AEPD) and the public entities and private companies, carried out these strategies urgently and in an exceptional way [3,4].
The survey was generated by a group of professionals from the public company with the aim of rewiewing and showing regarding the new strategies applied by the professionals who make up the overall clinical trial monitoring environment.
The results show that taking into account the demographic characteristics of our respondents, a frequent profile of Clinical Research Associate with an average age between 31-35 years is observed (both in public companies and in private industry)
The CRA profile is well defined by the current legislation and standards of good clinical practice, that describe them as professionals with the necessary clinical competence to directly monitor clinical trials, chosen by the sponsor and a direct link between the research team and the sponsor [5]. The degree of specialization of each CRA is established by the following items: their years of experience, their biosanitary academic training (such as graduates or degrees in Biology, Pharmacy, Biotechnology, Chemistry, Medicine, Nursing) [4], the training courses in good clinical practice, the performing of University masters of specialization in clinical trials [5,6].
Regarding the tasks where the clinical trial follow-up activity is carried out, it is observed that the main points are the Spanish cities with the largest population such as Madrid or Barcelona. This is due to the fact that the majority of the CRA profiles have a great geographical mobility, so the recruitment of these profiles is usually done in large population cities with good ways of communication with other Spanish cities
If we focus on the general information about monitoring, each study and its promoter (whether talking about public or private entities), establishes a monitoring plan. This plan establishes the frequency of: visits, protocol data reviews, checking of treatment adherence together with the Normalization Process Theory (NPT) of the centres and the sponsor.
The main activity of monitoring is defined as: “ the act of following a clinical trial development, and ensuring that it is carried out, filed and published in accordance with the Protocol, the NPTs, the GCPs and current regulations ”(ICH GCP 1.48).
In order to carry out this activity, the documentation sources must be reviewed (which in most cases corresponds to the electronic or paper-based medical history) and they have to be compared with the data included in the Electronic Case report form (eCRF) by the study coordinator.
When there are discrepancies between the documentation source and the eCRF, queries or findings arise. This data verification activity is supported by the fact that the eCRF comply with the quality standards and the regulations established for the management of clinical data and the regulations regarding security and confidentiality of clinical documentation [12].
When we analyzed the information sent by our respondents, we found out that not all monitors have had access to the documentation sources, therefore, the number of remote visits were increased. As they also reported, this type of visits was no frequent before the pandemic, therefore professionals have had to adapt to this situation in order to continue their activity monitoring clinical trials. The main limitation reported related to this change to remote activity was related with data security and confidentiality. Most public hospitals do not allow remote access for external professionals.
At the beginning of the COVID-19 pandemic, the Spanish Medicines Agency (AEMPS) issued a document of exceptional measures applicable to clinical trials to manage the emergency problems derived from the COVID-19 [3]. The main objectives of this document were related to maintain the health system capacity to reduce the risk of infection and to give recommendations to professionals to adapt to the epidemiological evolution as determined by the Ministry of Health.
The highlights of this document are:
Point 1: Related to program face-to-face visits of patients in a clinical trial, this document raised the option to consider postponing the visits or transforming them into phone calls. This fact is consistent with the data found in our survey, where the respondents informed that a paralyzing was observed in the end-of-trial visits and a decrease in the performance of initiation visits.
Point 2: Recruitment of new patients, assessing the risk benefit versus interrupting the recruitment of patients and even with the suspension of treatments.
Point 3: Access to trial treatment, to guarantee access to treatment to the patient included in a clinical trial. In our survey, the subjects reported in most cases that, due to the COVID-19 pandemic, they have proceeded to send medication at home to reduce the risk of contagion of the patients as well as they had to verify adherence to treatment through other ways.
Point 4: Informed consent, allow consent orally and before a witness, whenever the situation allows, documenting this fact in the patient's medical record and ratifying it in writing by signing the patient and the researcher.
Point 5: Monitoring visits, priority is given to centralized and remote visits of the centers that do not entail an overload of tasks for the center's staff.
This change in the monitoring modality has been carried out in private and public companies, establishing different communication channels based on: e-mail, teleconference and telephone calls. The results of our survey did not find significant differences between public and private entities in relation to this issue.
Nevertheless, in the assessment of the access to documentation sources in pandemic times, we found significant differences between public and private companies where most professionals have had access to electronic medical histories directly or through anonymized documentation.
In relation to this point, the AEMPS only allows remote access for “clinical trials that investigate the prevention or treatment of COVID-19 and for the final preparation of data prior to closing the database of pivotal trials investigating treatments for serious diseases without therapeutic alternatives. In any case, it will be done with the safeguards and precautions indicated in the EU guidelines and therefore will require the prior approval of each center with the approval of the data protection officer of the same [8-12].
In fact, some of the participants knew the reasons why they had not been able to access the patients' medical records as shown in Table 2.
Related to this section, the Spanish Data Protection Agency (AEPD) was consulted. The AEPD, according to the current legislation on confidentiality and the AEMPS guidelines, provides favorable information to carry out remote monitoring activities. In order to carry out this activity, an addendum to the contract will be made according to the AEMPS criteria and a confidentiality agreement must be signed of the by the monitor.
Point 6: Transfers of patients from one center to another, by agreement between the centers and acceptance by the Drug Research Ethical Committee (CEIm from Spanish: Comité de Ética de Investigación con medicamentos), in addition to complying with the sending of all the clinical documentation necessary to carry out the monitoring of the patient.
Point 7: Notification to the CEIm and the AEMPS.
The strategies developed and applied in this article offer new perspectives for the approach to CT monitoring, for which official authotities and public hospitals must adapt to the situation so that professionals can carry out their work with the rigor and quality that characterizes CT monitoring.
In the end, these data indicate that monitoring tasks are necessary and that public centers have to adapt to exceptional situations such as health emergencies that make face-to-face attendance impossible in order to maintain the follow-up of the clinical trial. Some of the recommendations that can be made are controlled remote access to the patients included in the trial, procedures that must be standardized by the different health institutions to comply with data quality [7,8,9] and current legislation [10,11].
The general conclusions reported from this survey carried out in professionals involved in the management and monitoring activities related to investigation and CT are summarized it in the following tables:
A total of 78% from the subjects indicate that the most relevant positive aspect is the costs saving due to resource optimization.
However, the 73.6% of the participants emphasize insufficient communication with the research team and, more importantly, 78.2% indicate that they do not have access to the medical records, i.e., to the source documents.
Generalisability
There have been many press reports on remote monitoring in Spain and even in pharmaceutical companies' own journals, but to date no surveys of professionals working in the clinical trial sector have been found.
This article represents with qualitative and quantitative data the situation experienced in clinical research by personnel belonging to public and private institutions during the pandemic. In comparison with other publications, the profile of the study coordinator as a communication link between the principal investigator and the monitor is observed as an advantage to improve the follow-up of the CT.
Thanks to the collaboration of the coordination staff of the SCReN (Spanish Clinical Research Network) platform of the Hospital Clínico San Carlos, Emilio Vargas (Coordinator, specialist in clinical pharmacology), Sara Muñoz (Quality Manager) and Gema Pino (Project Manager) and the other clinical research units that make up the platform: Hospital Virgen del Rocío, Instituto Investigación Sanitaria de Granada (FIBAO), Instituto Maimonides de Investigación Biomédica de Córdoba (IMIBIC), Fundación para la Investigación de Málaga en Biomedicina y Salud, Instituto de Investigación Sanitaria Aragón, Fundación Canaria de Investigación Sanitaria (FUNCANIS), Hospital Universitario Marqués de Valdecilla, Instituto de Investigación Biomédica de Salamanca (IBSAL), Hospital de la Santa Cruz y San Pablo, Fundación Instituto Investigación Germans Trias i Pujol, Hospital Clínico y Provincial de Barcelona, Instituto de Investigación Biomédica de Bellvitge (IDIBELL), Instituto de Investigación Hospital Universitario Valle de Hebron, Idiap Jordi Gol, Instituto de Investigación Sanitaria Incliva, Instituto de Investigación Sanitaria Hospital la Fe, Centro de Investigación Clínica del área de Badajoz, Instituto de Investigación Sanitaria de Santiago de Compostela, Instituto de Investigación Biomédica de A Coruña (INIBIC), Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IDISSC), Hospital Ramón y Cajal, Instituto de Investigación Sanitaria Gregorio Marañón, Hospital de la Princesa, Instituto de Investigación Hospital Universitario La Paz, Instituto de Investigación Hospital 12 de Octubre, Hospital Puerta de Hierro, Instituto de Investigación Sanitaria Fundación Jiménez Díaz, Hospital Virgen de la Arrixaca, Clínica Universitaria de Navarra, Instituto de Investigación Sanitaria Biodonostia, Biocruces. And to the coordinators of studies that promote independent clinical research.
Funding
No funding sources have been received for this article. It is a non-commercial study carried out by professionals working in public institutions such as IDIVAL.
Citation: Lavin-Alconero L, Nogueiras-Alvarez R, Fernandez-Lanas T, Garrido Jc, Burunat L, Gonzalez-Samperio C, et al. (2021) New Strategies for Monitoring Clinical Trials during the COVID-19 Pandemic: Descriptive Cross-Sectional Study. J Clin Trials. 11:485.
Received: 10-Sep-2021 Accepted: 24-Sep-2021 Published: 01-Oct-2021 , DOI: 10.35248/2167-0870.21.11.485
Copyright: © 2021 Lavin-Alconero L, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.