Journal of Aeronautics & Aerospace Engineering
Open Access
ISSN: 2168-9792
ISSN: 2168-9792
Research Article - (2017) Volume 6, Issue 4
In this paper considered a new method for controlling the thermal stratification of oxygen in a cylindrical rocket tank. The tank is cooled outside with liquid nitrogen, and then the tank begins to fill with oxygen. The walls of the tank are covered inside with a material with certain thermophysical properties and then polished.
<Keywords: Oxygen in the tank; Temperature stratification; Convection; Counteraction to stratification; New methods
As an oxidizer for liquid rocket engines (LRE) of modern launch vehicle (LV) is the most widely used (and will be used in the foreseeable future) liquid oxygen [1]. Suffice it to list LV "Zenit", "Mayak" (Ukraine), "Cyclone-4M"; "Antares" (US, Ukraine); "Atlas-V", "Falcon-9" (US); "Arian-V" (ESA); "H-1" (Japan); numerous rocket of family "Soyuz-2", "Angara" (Russia); KSLV-2 (Republic of Korea) and others.
In particular it should be noted that all these LV use cylindrical tanks without thermal insulation with liquid oxygen on different temperatures (from -183°C to -207°C). Tanks in the pre-start preparation after closing the drain valve (DC) and during the flight are heating under external thermal flows and, in its turn, are heating liquid oxygen. Under the influence of gravitational forces warmed layers of fuel float along the cylindrical walls of the tank top and accumulate there [2]. This is socalled thermal stratification of oxygen in the tank. At the input to the engine inlet device of LRE this oxygen layer enters at the end of engine work, on the throttling section. To illustrate the above, on Figure 1 shows the temperature of liquid oxygen at the inlet of the first stage engine LV "Titan" (USA) by flight time.
The temperature stratification of oxygen extremely negative phenomenon, even if it is only a few degrees. To understand its influence on the parameters of LRE consider the equation to determine the required gas pressure in the tank (рб) on temperature at the entrance to the oxygen intake device or the pump (tвх) (which is determinative in this case):
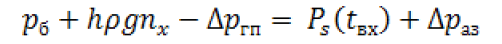
Where is:
h-height of the column of liquid in the tank at the entrance to the engine (over the intake device);
ρ-Average mass density of fuel;
g-Acceleration of gravity;
nx-The current value of axial load;
ΔpΓπ -Loss of pressure in the intake unit and expenditure pipeline;
Рs-Fuel saturated vapor pressure;
Δpaa -Anticavitation margin of pump (or intake device).
As seen from the equation, the higher fuel temperature at the inlet of the engine- the more value should be the first member in his right side. For example, heating of liquid oxygen by 1 degree in the temperature range 80 ÷ 96 K corresponds to an average increase of its vapor pressure at ~0,15 bar. In other words, in this case, for work pumps without cavitations is necessary to increase gas pressure in the tank to ~0,15 bar per every degree of warming up of oxygen or increase heat remains oxygen in the tank.
Figure 2 shows a typical minimum required gas pressure in the tank for a modern cylindrical oxidizer tank upper 1 stage of LV.
Figure 2: Typical changes required gas pressure in the upper tank of oxidant by work time of LRE (0 time - the beginning of the LV movement). Рмн.з-Required gas pressure in the tank during launch LRE; Рмнпр. Required gas pressure in the tank in terms its durability (stability); Рмнзу-Required gas pressure in the tank to ensure work without cavitation of the intake device.
As seen from the graph, the determinative calculation case for the gas pressure in the tank, in this case, is to ensure non- cavitation work of intake device at the end of LRE work ~110 s of flying. Fuel level (h) already small, overload (nx), before shutting down the engine, insignificant. Therefore, exceeding the gas pressure in the tank, the minimum required pressure (in this case- on condition non- cavitation work of intake device) can be achieved only by increasing the gas pressure in the tank.
However, to increase the gas pressure in the almost empty tank (for example, tank volume of oxidant on RD-171 M~200 m3) is rather complicated problem. If the pressurization system (PS) uses high pressure balloons, then the gas pressure in the balloons at the end of LRE work is small. When throttling LRE expenditure of coolant and its temperature at the inlet of the heat exchanger are reduced. Accordingly, when the reduced expenditure of helium from balloons then falling its temperature at the inlet to the tank.
If the tank pressurization system is evaporator (uses the evaporation of oxygen) or gas generator (on liquid propellant components), on the final section of LRE work (throttling) falling expenditure of fuel components (working substance of pressurization in the unregulated PS) and pressure in points of their collection. In both cases it is necessary to significantly increase the consumption of the pressurization working substance by introducing additional units of automation and increase flow area of the pressurization hot line through pressure reduction at the entrance. Therefore, finding ways to reduce the temperature of the upper layers of the fuel component in the tank is very promising way to increase the efficiency of pressurization system and rocket complex as a whole.
Analysis of recent research and publications that started solving this problem and to which are based authors. In the present experimental study [3] showed that for cylindrical fuel tanks without thermal insulation, despite the hot pressurization, determinant (for warming up the oxygen in the tank) is the external heat flow to its cylindrical surface.
In this case, it is accepted that warmed layers float to the surface and do not mix with each other, like each other layering [2]. In paper [4] shows that only during the pre-pressurization tank (drain valve closed) upper layer of oxygen in the 1 stage LV tank (with lengthening~5.6) warm up to 2°C. This is a significant value.
There are known means to eliminate the temperature stratification of fuel in tank [5]. For example, use different circular bulkhead over the entire height of the tank that breaks the natural convection flow of oxygen along the walls of the tank. The disadvantage of such methods is the complexity of the tank design and complexity of its manufacturing technology.
Also known method which includes the supply of cold helium by small flow from the bottom up through the tor collector ("gas-lift") [6]. The cold helium in a bubble floats up and mixes the heated and cold layers of liquid oxygen. Reduce the temperature of upper layer of the propellant component. The temperature of oxygen at the inlet of the engine close to average mass temperature. The disadvantage of this method is that it is aimed to eliminate the effects of heating propellant, and not the cause of its occurrence. Collector, additional helium, balloon for its storage-burden LV. Ideally, this method can reduce heating of the upper layer of oxygen for a few degrees (also very important), but do not reduce heating to a minimum. There is also another problem issue– a phenomenon possible movement of gas bubbles down under the influence of certain frequencies in the liquid phase. LRE, as known, generate the widest range of different frequencies.
Research task formulation. Purpose this work is study a new method for eliminating the temperature stratification in the tanks of liquid oxygen for rocket propulsion systems. Herewith, it should minimize heating of only the upper part of the oxygen in tank, which creates problems. Analysis of Figure 1 shows that it's advisable to eliminate heating of oxygen in tank enforce measures only to part of the fuel that enters to intake device after ~110 sec of LRE work. Oxygen that is consumed before this time does not create any problems to ensure the required gas pressure in the tank and spend resources on it just impractical. Also proposed method should not burden the fuel tanks, should be implemented at the existing level of science and technology, and will not require the development of new difficult technologies.
Consider a typical way of filling fuel tanks with oxygen for propulsion system of LV. On the expenditure pipeline from start complex fed liquid oxygen that meets to standards (DSTU– State Standard) the chemical and mechanical purity for rocket fuel. The liquid oxygen in tank is cooling the tank structure and boiling. The temperature of the tank structures is close to the temperature of liquid oxygen in a tank, but it is always warmer. During the filling the tank, a pair of oxygen from cryogenic temperatures release through open drain valve to the atmosphere. Cold vapor of oxygen sink to the ground launching pad and significantly increasing the concentration of oxygen there. This creates a highly fire risk situation.
On completion the tank fueling the drain valve is closing. Oxygen begins to warm up and take place temperature stratification. After prelaunch preparation LRE is starting. Liquid oxygen under the influence of aerodynamic heat flow directed to the walls of the tank continues to heat up in the boundary layer and come to the oxidant surface [7].
The disadvantages of this method are:
1. Significant value of expansive pure oxygen consumed for the cooling structure of tank;
2. On the launch pad during the filling procedure is formed highly concentrated oxygen environment. When an accident happens, that unfortunately occur, extinguish the fire in this atmosphere on launch pad, very difficult;
3. At the launch, after drain valve closing begins temperature stratification of the oxygen in the tank
The solution of the problem is planned as follows [8]. Before filling the tank and before the start of LV cooling tank walls by neutral liquefied gas, such as nitrogen, from its outside achieving a temperature that is below the temperature of the component of fuel in the tank at the time of launch. From the outside the tank can be cooling by untreated liquid nitrogen, giving it top-down coaxially to tanks walls until the launch LV. To reduce consume of nitrogen it is advisable to supply nitrogen to coaxial gap between the tank and special ground casing. To reduce nitrogen consume it is advisable to supply nitrogen to coaxial gap between the tank and special ground casing.
In this case we have following physical process of the movement of oxygen in the tank. At the tanks top is formed freely convective movement of oxygen down along the cold walls. At non-cooling part of tank layers of heated oxygen rise up, meet an obstacle, mix and coming into the center of the tank (Figure 3).
The effect will be enhanced if used supercooled nitrogen. In this case, is achieved significant saving of liquid oxygen at fuelling procedure. The temperature of the top of the oxidizer tank construction will be lower by 10 ÷ 15 °K than oxygen tank temperature. This makes it possible to eliminate heating of the volume of oxygen that we are interested in the pre-launch and reduce it in the first phase of flight. Cooled gaseous nitrogen in large quantities on the surface of the launch pad also improves fire safety.
The presence of liquid nitrogen on the launch pad is not a challenge.
Since the early fifties (missile R-5) and to this day (series of LV "Soyuz-2") tones of liquid nitrogen filled on the start in rocket propulsion system for pressurization of fuel tanks.
Using technological cheapest liquid nitrogen (with air addition) instead of highly purified liquid oxygen significantly reduces cost of oxidant tank cooling. Another advantage-in advance lowering the temperature inside the tank before supply liquid oxygen reduces the chance of an explosion if there were violated the technology requirements (left traces of organics, such as oils, after milling of internal surfaces of tank).
Also, a positive effect can be achieved by reducing the amount of vaporization centers on the walls of the tank, for example by polishing its internal surfaces [8].
This conclusion follows from the empirical equation of Han and Griffith for the heat flux density [9]:
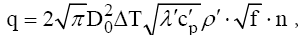
where, D0- Initial diameter of the bubbles;
ΔT - Temperature driving force;
λ',c'p,ρ'-Thermal conductivity, heat capacity and density of the liquid;
f- Bubble frequency;
n - Density of vaporization centers.
A similar result can be obtained if the inner surface of the fuel tank, or part of it, be covered material, which has complex (cpλρ) than the same complex for material of the tank walls. It is known that with the decrease of complex (cpλρ) eases the intensity of heat transfer [7].
In summary, the proposed technical solution can: reduce mass of pressurization system by reducing the temperature of liquid oxygen in tank; increase fire safety; reduce the cost of oxidizer tank cooling. To confirm the proposed method should make the modeling test.
Conclusion from this study and prospects for further development in this R and D direction. The proposed method of pressurization of the fuel tanks with liquid oxygen theoretically ensures reduction:
-The mass of helium for pressurization of tank;
-Gas pressure in the tank (mass of tank and pressurization system);
-Expenditure of expensive rocket oxidizer during refueling and improve the fire safety on the launch pad.
The prospects that the proposed method opens– namely a significant improvement of safety and reliability Launch Complex, certainly deserving of its experimental research.