Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Review Article - (2015) Volume 3, Issue 2
The natural cycle of nitrogen involves several biological and non-biological process including: mineralization, nitrification, denitrification, nitrogen fixation, microbial and plant uptake of nitrogen, ammonia volatilization, leaching of nitrite and nitrate and ammonia fixation. Nitrogen exists naturally in the environment and is constantly being converted from organic to an inorganic form and vice versa. Production of commercial fertilizer adds up to the natural source of nitrogen. The main source of nitrogen include: atmospheric precipitation, geological sources, agricultural land, livestock and poultry operations and urban waste. Agricultural emissions show a strong increase due to the application of fertilizer to agricultural soils, grazing of animals and spreading of animal manure. Emissions from agricultural practices and animal manure wastes are the major source of nitrogen pollution in surface and underground water. Soil erosion and runoff from fertilized land as well as domestic and industrial wastes contribute to the enrichment of lakes and streams with nutrients. Nitrates concentration exceeding certain limits in drinking water is toxic to animals and humans, especially infants. Nuisance of algal bloom and fish kills in lakes and rivers occurs due to eutrophication. Obnoxious colours and smells are developed as a result of organic matter decay and are destroying the natural beauty of the environment. The water born contaminants affect human health from both recreational use of contaminated surface water and from ingestion of contaminated drinking water derived from surface or ground water sources. The methods for abatement of nitrogen pollution must follow multi pathways. First, the source and amount of pollution must be detected and defined. Second, the possible ways to treat animal and domestic wastes should be carefully investigated. Third, better agricultural practices should be developed that include: proper storage and application of slurry and solid manure, rapid incorporation of slurry and solid manure into the soil, use of band spreading machineries such as trailing house and trailing shoe and sub-surface applicators, use of specifically made round covers fitted to above ground tanks and slurry lagoons, applying fertilizers during periods of greatest crop demand at or near the plant roots in smaller amounts with frequent applications, using multiple cropping systems such as using crop rotations or intercropping to increase the efficiency of nitrogen uses and changing current livestock production techniques.
Keywords: Nitrogen cycle; Organic nitrogen; Nitrogen dioxide; Ammonium; Nitrification; Volatilization; Denitrification; Nitrogen fixation; Leaching; Fertilizer; Manure; Air; Water; Soil
Water is an indispensable resource on which human existence depends. About 73% of the fat free body weight in healthy human adults is water which is equivalent to 60% of body weight for non-obese subjects [1]. Although three quarters of the earth is covered with water, only one percent of which is fresh water that is considered safe for use to meet our daily needs. Water that cannot be used because it is polluted: (a) reduces present and future supply, (b) inhibits local, regional and national economic growth, (c) poses known and unknown dangers to the public health, (d) curtails expansion of recreational activities and (e) further dislocates the already badly disrupted balance of nature [2].
Water pollution may be defined according to the usage of water and the presence of certain constituents and their effects on human health and the surrounding environment. One of the widely accepted definition of water pollution is “the introduction into water substances of such character and in such quantity that alter its natural quality as to impair its usefulness or render it offensive to the human senses of sight, taste or smell” [3,4]. A substance may not appear by itself as pollutant, but may indirectly (by its effect upon other materials or living organisms) lead to an offensive effect. The determination of whether a certain constituent creates a nuisance, impairs the usefulness of the water or interferes with nature’s balance depends on the subsequent use of the water. Water can be polluted from many human activities and pollutants not only reduce the water quality and quantity but may act as harmful media to other living organisms. Polluted water can contain excess nutrients, pathogens, toxic materials and dangerous chemical which can affect human health, destroy marine species and disturb the ecological cycle [5,6].
Nitrogen in its elemental form is the major component of the air constituting about 78% of the gases in the earth atmosphere. There are also different nitrogen gaseous compounds that exist in the atmosphere including NH3, NO and N2O. The importance of nitrogen to life is that it constitutes (with carbon, hydrogen and oxygen) the major part of proteins of all living materials. All living organisms, except few microorganisms (nitrogen fixing bacteria, algae and fungi), cannot use N2 as a source of nitrogen and need different forms of fixed nitrogen (NH4 and NO3 for plants and organic nitrogen for animals and humans) as a supply for their requirement of protein synthesis.
The elemental form of nitrogen (N2) is very unreactive and hard to extract from the environment although there are approximately five billion metrics tons of nitrogen around the earth [7]. Some forms of nitrogen are considered poisonous to plants, animals and humans if they exceed certain concentration in their environment. The presence of higher quantities of fixed nitrogen (NH4 and NO3) in the water or soil will encourage vegetative growth of plants beyond the favourable level. Nitrogen pollution means pollution due to the fixed forms of nitrogen (bonded with carbon, oxygen or hydrogen) also known as reactive nitrogen [8].
Nitrogen fixation is being done in nature in several ways and passed to the rest of living organism. Nitrogen will return back to the soil after the death of these organisms through the activities of soil microorganisms which provide the nitrogen in its ready form again to plants and then to animals and humans. Certain amounts of this fixed nitrogen are liable to be lost to the atmosphere in elemental form. However, the significant increase in human population increased the demand for food which leads to mass production of synthetic nitrogen fertilizers for agricultural activities. Human activities such as production and use of commercial fertilizer, production and use of fossil fuels in industrial processes, energy generation and transportation altered the nitrogen cycle and caused disturbance to the total environment (Air, soil and water) [9].
The importance of nitrogen from the standpoint of fertility of the soil has long been recognized and our knowledge concerning the nature, distribution and transformations of nitrogen compounds in soil is extensive. A schematic diagram depicting the cycle of nitrogen in nature is shown in Figure 1. The nitrogen cycle includes several biological and non- biological processes. The biological processes are: ammonification/ mineralization, nitrification, denitrification, nitrogen fixation, nitrogen assimilatory reduction and microbial synthesis of ammonium and organic nitrogen into microbial cells, plant uptake and conversion of ammonium and nitrate nitrogen into plant proteins. The non-biological processes are ammonia volatilization, leaching of nitrite and nitrate nitrogen to ground water, ammonium fixation into soil clay minerals, precipitation of nitrate and ammonium nitrogen.
Mineralization (or ammonification) of soil nitrogen is the term used for the process by which nitrogen in organic compounds (CaHbOcNd) is converted by soil microorganisms into ammonium ion (NH4+) as follows [10,11].
Complex organic nitrogen → Ammonium (1)
The soil microflora typically produces ammonium from organic compounds when they set free more nitrogen from the organic matter on which they are living than they can assimilate into their own protoplasm. This concept of the ammonium production (being the nitrogen waste product in the conversion of organic matter into microbial tissue and use of vital energy) is fundamental for understanding the effect of adding different types of organic matter on the mineralization of nitrogen in the soil [12]. Thus, when an animal protein (such as dried blood) is added to a soil, about 80% of the added nitrogen is liberated as ammonium and the remainder of the nitrogen is retained in microbial tissue. However, by increasing the quantities of a carbohydrate, such as cellulose mixed in with the protein, the amount of microbial tissue that can be built up is increased, with the consequence that the proportion of nitrogen liberated as ammonium decreases until the ratio of carbohydrate to protein reaches a value of about 5:1 when all the nitrogen in the dried blood is needed by the microorganisms [13].
The accumulation of ammonium in the soil is affected by: (a) the rate of mineralization of organic nitrogen in the soil, (b) uptake of ammonium by microbes as a source of nitrogen for growth, (c) uptake of ammonium by plants as a source of nitrogen for growth, (d) volatilization of ammonia, (e) nitrification (biological conversion of ammonium to nitrate), (f) loss of nitrate by leaching (which increases the rate of nitrification) and (g) plant uptake of nitrate as a source of nitrogen for growth (which increases the rate of nitrification). Therefore, a low concentration of ammonium in the soil does not indicate low mineralization and may indicate high rates of nitrification, volatilization or microbial and plant uptake. Nonetheless, net mineralization will be directly affecting the organic nitrogen content in the soil and the availability of carbon for microbial growth. Thus, vegetation with high C: N ratio will result in a low rate of nitrogen mineralization [14].
Nitrification
Nitrification is the oxidation of ammonium nitrogen to nitrites and nitrates. It is the result of metabolism by chemoautotrophic (or chemolithotrophic) organisms. The two groups of organisms that are considered to be the primary nitrifying bacteria are Nitrosomonas Sp. and Nitrobacter Sp. Nitrosmonas carry out the oxidation of ammonium to nitrite to obtain energy (E) and Nitrobacter oxidizes nitrite to nitrate for the same purpose. The general oxidative processes involved can be represented by the following equations [15,16]:
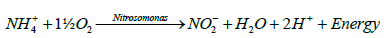 (2)
(2)
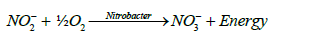 (3)
(3)
Both genera use CO2 as their sole carbon source for growth as follows, as they are obligate autotrophs and strict aerobes [17].
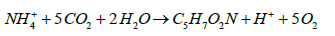 (4)
(4)
Nitrifying bacteria have a low activity level compared with heterotrophs. For example, it takes Nitrosomonas nearly an hour to produce twice its weight in NO2 whereas some heterotrophic bacteria produce one thousand times their own weight in products in the same time. Under aerobic conditions, the endogenous respiration of the organisms results in the breaking down of cellular nitrogen and its release as ammonium [18,19].
Denitrification
Denitrification (or nitrate reduction) is a more complex and less understood process than nitrification. Nitrification and denitrification are redox processes involving nitrogen compounds to obtain energy. There are two processes of nitrate reduction: assimilatory and dissimilatory.
Assimilatory nitrate reduction: Assimilatory nitrate reduction (ANR) is one of the main processes in the nitrogen cycle in which nitrate (NO3-) is used as nitrogen source for the growth of new cells. At first, the nitrate (NO3-) is incorporated into the cells by a high affinity transporter which is then reduced to nitrite (NO2-) by the enzymes nitrate reductase which is further reduced to ammonium (NH4+) by the enzyme nitrite reductase. The ammonium which is produced in incorporated into the carbon skeltons by glutamine synthetase or glutamine synthase pathway as shown in Figure 2 [20].
Bonete et al [20] reported that the assimilatory nitrate reduction takes place in three steps: (a) nitrate (NO3-) uptake, (b) reduction of nitrate (NO3-) to nitrite (NO2-) and (c) reduction of nitrite (NO2-) to ammonium (NH4+). In the first step, the nitrate (NO3-) uptake into the cells takes place using ATP-dependent ABC transporter. In the second step, after the nitrate is being imported into the cells, it is reduced to nitrite (NO2-) by ferredoxin dependent assimilatory nitrate reductase (NR). In the third step, the nitrite is reduced to ammonium (NH4+) by ferredoxin dependent assimilatory nitrite reductase (NiR). The process is described as follows:
 (5)
(5)
Dissimilatory nitrate reduction: Dissimilatory (or respiratory nitrate) reduction is the process in which nitrate (specifically the oxygen in nitrate) serves as the terminal hydrogen acceptor in energy yielding reactions. It has been shown that nitrate reduction by this process closely follows the same steps as when molecular oxygen is used. It is tied in with the cytochrome system for electron transport. Thus, when the dissolved oxygen level drops to low levels for the aerobic metabolism of facultative organisms they can turn to nitrate reduction for oxygen fairly easily. The term denitrification is assigned to dissimilatory nitrate reduction. The general simplified equation for the denitrification process is postulated as follows [21-24]:
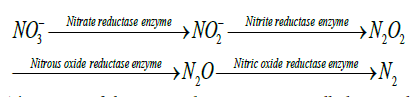 (6)
(6)
The nature of the intermediates is not generally known although many workers have suggested N2O2 and N2O which themselves may involve other complicated reactions [14,25]. There are a great many genera of bacteria that can accomplish denitrification among them are Pseudomonas Sp., Thiobacillus denitrificans (an autotroph) and Micrococcus denitrificans. The presence of dissolved oxygen has been found to inhibit the denitrification process to various degrees. This point out the need for low or zero dissolve oxygen levels before denitrification can occur [26,27].
Nitrogen fixation
Although atmospheric nitrogen (N2) is abundance in the atmosphere, it is not in a ready from of nitrogen to be used by most organisms. Strong triple bond which links the nitrogen element in N2 is hard to break. In Harber process, in which chemically synthesized nitrogen is formed, red hot magnesium or a catalyst is used at elevated pressures and temperatures to make N2 reactive [28]. However, in the biological nitrogen fixation process, nitrogen is fixed by the microorganisms that are capable of breaking the bond at ambient temperatures and pressures and is called diazothrophic [28,29]. The dizaotrophs are available in soil both as free living and in symbiotic association with plants. Diazothrophic microorgainsms use the enzyme nitrogenase to carry out the fixation process. There are about 100 species of enzymes but they are very similar in their activities [28]. Nitrogenase reduces dinitrogen to ammonium (NH4+). The enzymatic reaction can be described as follows:
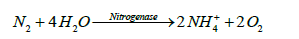 (7)
(7)
Soils can gain small amounts of nitrogen from the rain which falls on them. However, the most important natural process for increasing the nitrogen content of soils is nitrogen fixation by microorganisms living in the soil on and around the roots (Figure 3). A functional classification of the range of nitrogen fixing bacteria is soil is shown in Table 1 [30]. The estimates of nitrogen fixed by different microorganisms are shown in Table 2 [31]. Nitrogen fixation is inhibited in the presence of high level of available nitrogen (NH4+ or NO3-). The process is also controlled by N: P rations as phosphorous activate the gene for synthesis of [31]. Nitrogen can be fixed in the soil through symbiotic fixation and asymbiotic fixation. The rate of nitrogen fixation is related to the rate of photosynthesis (plant growth). A comparison between nitrogen fixation and photosynthesis is shown in Table 3.
| Type | Nitrogen fixing bacteria | Symbiont |
| Heterotrophs | ||
| Free living | ||
| Anaerobic | Clostridium | |
| Microaerophilic | Frankia, Azospirullum | |
| Aerobic | Bradyrhizobium, Azotobacter, Derxia | |
| Root-associated | ||
| Microaerophilic | Azospirullum, Herbaspirullum | |
| Endophytic | Acetobacter | Sugar cane (Saccharum sp.) |
| Symbiotic | Frankia | Casuarina sp. Alnus sp. |
| Rhizobium, Bradyrhizobium | Many legumes | |
| Azorhizobium | Sesbaniarostrata | |
| Autotrophs | ||
| Free living | ||
| Anaerobic | ||
| Microaerophilic | Rhodospirillum, Bradyrhizobium | |
| Aerobic | Cyanobacteria | |
| Symbiotic | Cyanobacteria | Fungi (lichens), Cycads |
| Anabaena azollae | Azolla sp. | |
| Bradyrhizobium | Aeschynomene sp. | |
Table 1: Classification of nitrogen fixing bacteria which contribute to agriculture [30].
| Nitrogen fixing system | Nitrogen (N2)fixed (kg N ha-1) |
| Free living | |
| Rice-blue green algae | 10-80 |
| Rice-bacterial association | 10-30 |
| Sugarcane bacterial association | 20-160 |
| Symbiotic | |
| Rice-Azolla | 20-100 |
| Legume-Rhizobium | |
| Leucaenaleucocephala | 100-300 |
| Glycine max | 0-237 |
| Trifoliumrepens | 13-280 |
| Sesbaniarostrata | 320-360 |
| Non-legume-FrankiaCasuarina sp. | 40-60 |
Table 2: Estimation of dinitrogen fixed by different nitrogen fixing systems [31].
| Parameters | Nitrogen fixation | Photosynthesis |
| Differences | ||
| Medium | In soil | Above soil |
| Substrate | N2 | CO2 |
| Delivery point | Root | Leaves |
| Machinery | Enzyme nitrogenase | Chlorophyll |
| End Product | NH4 | CH2O |
| Energy | Chemical | Solar |
| Organism | Bacteria, Fungi, Blue green algae | Plant, Algae |
| Organic-C | Decomposed | Produced |
| Similarities | ||
| By product | O2 | O2 |
| Need for P | For nitrogenase | For ATP |
| Rate | Affected by nutrients | Affected by nutrients |
Table 3: A comparison between nitrogen fixation and photosynthesis.
Asymbiotic fixation of nitrogen: Soils contain a number of free-living, nitrogen-fixing organisms such as bacteria (Azotobacter, Bejerinckia, some Clostridium, and Aerobacter, Achromobacter and Pseudomonas), blue-green algae and yeast which possess the enzyme nitrogenase needed for nitrogen fixation [32]. The processes take place in soils high with organic matter that provide a ready source of energy. Nitrogen fixation goes on more actively under conditions of poor rather than good aeration, when the level of available nitrogen salts is low [33]. Asymbiotic nitrogen fixing bacteria are extremely diverse occurring in nine subdivision of the eubacteria, four subdivision of archeabacteria. Free living prokaryotes such as diazotrophs which have the ability to fix atmospheric free nitrogen are ubiquitous in the soil. The ability of free living diazotrophs to perform nitrogen fixation depends upon various conditions such carbon, nitrogen and oxygen partial pressures. The diazotroph populations depend upon the C: N ratio which is potentially used as bioindicators of nitrogen status of the soil. The contribution of assymbiotic nitrogen fixing bacteria in terrestrial ecosystem is much higher than symbiotic nitrogen fixing bacteria [34-36]. It is estimated that free living prokaryotes are capable of fixing nitrogen in the range of 0 to 60 kg/ha/y.
Symbiotic nitrogen fixation: Certain leguminous plants and a few other non-legumes possess nodules in their roots. These nodules contain bacteria (belong to the genus Rhizobium) and fungi living symbiotically with the plant. They have the ability to fix nitrogen and provide the plant with nitrogen compounds while they receive their source of energy (carbohydrate) from the plant.
Some of the symbiotic nitrogen fixing bacteria such as Azolla in symbiosis with Anabaema azollae can fix 2-4 kg N/ha/day. There are other benefits of Azolla including: (a) they can be used as weed suppressor, (b) potassium (K) scavenger from floodwater, (c) can be used in animal feed and fish feed, (d) phosphorous (P) scavenger in sewage treatment and (f) suppressor of ammonia volatilization [31]. Heterotrophs such as Azotobacter chroococcum and Azotobacter vinelandii are excellent symbiotic nitrogen fixing bacteria but they have strict requirement for neutral pH conditions and, therefore, their availability in tropical soils is very rare. However, other symbiotic bacteria such as Beijerinckia indica, Bejierinckia fluminensis, Azospirullum sp. and Herbaspirullum sp. are more tolerant to low pH and have wide range of ecological adaptation to fix nitrogen [30].
The nitrogen uptake by free living bacteria and blue green algae takes place in soils high in organic matter that provided ready source of energy. The reduction of N2 to NH4 has large metabolic costs (respiration of organic carbon in the soil) as follows:
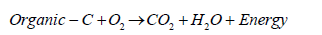 (8)
(8)
The energy cost of nitrogen fixation links the biogeochemical process to the availability of organic carbon provided by photosynthesis. Asymbiotic are an important source of nitrogen for terrestrial ecosytems. The requirement of molybdenum (Mo) and iron (Fe) as structural components of nitrogenase links nitrogen fixation to availability of these elements in nautral ecosystems. Phosphorous is also required for the production of the enzyme nitrogenase.
The symbiotic association between fungi and higher plants are found in most ecosystems and is important for the nutrition of plants. Many trees harbor the ecotrophic mycorrhizal fungi which form a sheath around the active fine roots and extend their hypae into surrounding soil. Because of their large surface area, fungi obtain soil nutrient and transfer then to plants thereby contributing to weathering of soil minerals through the release of organic acids. Fungi in return, depend on plants for carbohydrate as source of carbon energy [37]. Nodulated legumes are used in cropping systems for centuries and they are used as primary source of food, fuel, fiber and fertilizer to other plants or to enrich the soil with nitrogen [31]. After removing the crop from the field, plant nodules will be a good source of nitrogen in the soil.
Microbial uptake of ammonium
The ammonium uptake across the biological membranes is generally facilitated by ammonium transporters (Amt). The ammonium transporters (Amt) are a class of membrane-integral transport proteins which are found in archaea and bacteria and even in eukaryotes [38,39]. The ammonium produced from nitrite reduction is assimilated by several microorganisms using glutamine synthetase-glutamate synthase (GS-GOGAT) pathway or glutamate dehydrogenase (GDH) pathway. The GS-GOGAT pathway requires ATP but has higher affinity towards ammonium. The GDH pathway does not require ATP and is less effective in growing cells in nitrogen limited conditions [20].
Most microorganisms use both pathways for ammonia assimilation. However, when the microorganisms which are able to assimilate nitrate are exposed to ammonium, the nitrate assimilation by the cells are drastically inhibited. Ammonium inhibition can be long or short term. Prolonged incubation of cells to ammonium can lead to repression of transporter genes and nitrate reductase (Nas) and nitrite reductase (Nir) enzymes. Shorter incubation of cells in ammonium inhibits nitrate and nitrite uptake without affecting the genes or enzymes [40].
Plant uptake of ammonium and nitrate
Most of the plant species absorb and assimilate nitrate (NO3-), ammonium (NH4+), urea and amino acids as nitrogen sources, but the specifity of these sources vary with different plants. Crawford and Glass [41] reported that the optimal ratio of nitrate : ammonium is 3:1 for tomato roots and the growth is inhibited if the ratio of ammoium is increased, but for white spruce prefer more ammonium in the soil and some artic sedges prefer more amino acids in the soil. Both nitrate (NO3-) and ammonium (NH4+) possess some common features including: (a) both ions (NO3- and NH4+) are absorbed by the roots at low external concentrations, (b) there are high affinity transport systems (HATS) in the roots for both nitrate (NO3-) and ammonium (NH4+) (c) the HATS for nitrate is double the HATS for ammonium and (d) the influx of both ions is responsive to plant N status and is subject to diurnal regulation [42].
Meyer et al. [43] and Stitt [44] reported that plants, unlike bacteria or fungi use ammonium as a nitrogen source and show better growth in the presence of nitrate. Nitrate can get accumulated in the leaves of the edible plants or in drinking water and may affect both the environment and human health. After nitrogen uptake, nitrate is either stored in the plant root system or translocated to aerial parts via the xylem. High concentrations are usually found in the vacuoles as a nutrient source and plays an important role in the maintanence of osmoticum. Nitrate reduction in plants takes place both in roots and shoots. The nitrate reduction takes place in cytoplasm and the nitrite reduction takes place in plastids/chloroplasts. The reduction of nitrate to nitrite is catalyzed by the enzyme nitrate reductase (NR) enzyme. The nitrite after nitrate reduction is translocated to the chloroplast where it is reduced to ammonium by nitrite reductase (NiR) enzyme.
Ammonium obtained after nitrite reduction and also from photorespiration or amino acid recycling is assimilated in the plastid/ chloroplast by GS/GOGAT cycle. The glutamine synthetase (GS) fixes ammonium on a glutamate molecule to form glutamine. The glutamine formed reacts with 2-oxuglutarate to form two molecules of glutamate in the presence of glutamine 2-oxyglutrate amino transferase (GOGAT) enzyme [45,46]. There are two different forms of GOGAT enzymes present in plants: Fd-GOGAT and NADH-GOGAT. Fd-GOGAT uses ferredoxin and NADH-GOGAT uses NADH as electron donors, respectively. Fd-GOGAT is usually localized in the plant leaves, whereas NADH-GOGAT is localized in plastids of non-photosynthetic tissues such as roots, etiolated leaf tissues and companion cells. The ammonium formed in the plastids are converted to carbomylphosphate which is a precursor of citrulline and arginine in the presence of carbamoylphosphate synthase (CPase) as shown in Figure 4 [46,47].
Both NO3- and NH4+ are absorbed by plants in the form of amino group (- NH2). The availability of NH4+ or NO3- depends on the environmental conditions that affect the production of NH4+ and the conversion of NH4+ to NO3-. However, many plants species show preferences to NO3- over NH4+, although thermodynamic analysis suggests that the metabolic energy cost of reducing NO3 to NH2 is significantly greater. There are several reasons for plants showing preference towards NO3- over NH4+: (a) NH4+ interacts with soil cation exchange where NO3- is slightly soluble and mobile, (b) the rate of NO3- delivery by diffusion is higher than that of NH4+, (c) plant that uses NH4+ have to compensate for the difference in diffusion by investing more energy in the root growth, (d) uptake of NO3- avoids the competition that occurs in root enzyme carriers between NH4+ and other positively charged ions and (e) relatively high concentration of NH4+ is toxic to plants.
The amino group (-NH2) is attached to soluble organic compounds and the nitrogen absorbed by the roots is transported to the xylem in the steam (capillary system) as amides, amino acids or uried before its eventually incorporated into proteins in leaves. There are two types of amides: (a) metallic derivatives of NH4+ in which NH2 group is retained and (b) organic derivative. The following are examples of the different types of amides, amino acids and uride.
(a) Metallic Amides:
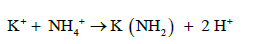 (9)
(9)
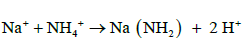 (10)
(10)
(b) Organic Amides:
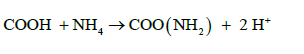 (11)
(11)
(c) Amino Acids:
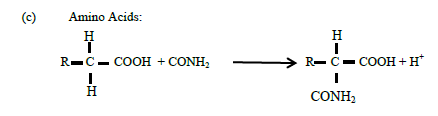 (12)
(12)
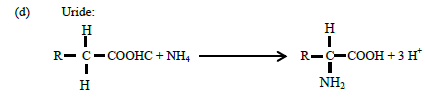 (13)
(13)
In case of NO3 uptake by plant, it is first reduced to amino group which is then interacts with CO2 to form amine. The amine interacts with organic acid to form aminoacid as follows.
a) 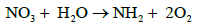 (14)
(14)
b) 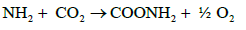 (15)
(15)
c)  (16)
(16)
Ammonia volatilizaiton
Ammonium exists in two forms: free or unionized form (NH3) and ionized form (NH4+). Ionised ammonium is soluble in water while unionised ammonia is volatile and could be easily removed from water. The process of volatilization carries the free ammonium from the water into the atmosphere. un-ionized ammonia is volatile and could be easily removed. Ammonium volatilization occurs when ammonium ions are present in an alkaline medium and gets dissociated into gaseous ammonia which then gets released into the atmosphere [48].
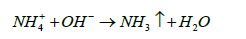 (17)
(17)
The overall process of ammonia volatilization could be comprised into four major steps: (a) conversion of iodized ammonium into free ammonium by dissociation, (b) diffusion of free ammonia to the waterair interface, (c) volatilization or releasing the free ammonium to the atmosphere at the interface and (d) diffusion of free ammonium from the water-air interface into the atmosphere which is carried out by mass transfer [48-50].
Various agricultural activities including livestock production and fertilizer application are the main sources of atmospheric ammonia emisson. Ammonia emission occur from livestock buildings, open feedlots and maure storage facilities as well as manure handling and application to the land. In livestock facilities, the urea present in the urine is broken by the enzyme urease which is present in the feces to release ammonia into the atmosphere. There are several factors affecting ammonia volatilization including: (a) manure type and characteristics, (b) temperature, (c) wind speed, (d) pH and (e) method and timing of manure application [51,52].
Rochette et al. [53] reported that the impact of urea application rate on the nitrogen loss in the form of ammonia (NH3) is variable as shown in Table 4 [54-64]. The large variability in the results reported from several studies has been due to the nonlinear response of the ammonia to variation in pH and the availability of ammonia in the soil. Ammonia volatilization occurs in a soil when the pH is high (>7.5) [48]. Sommer et al. [65] reported that pH affects the ammonia loss and is affected by climatic and soil conditons.
| Conditions | Soil pH | N rate (kg ha-1) |
NH3 loss (% appl. N) |
N placement | References |
| Laboratory | 9.0 | 15 | 56 | Surface broadcast | [54] |
| 30 | 48 | ||||
| 60 | 41 | ||||
| 120 | 34 | ||||
| 240 | 29 | ||||
| Field | 8.7 | 89 | 14 | Surface broadcast | [55] |
| 178 | 16 | ||||
| 255 | 20 | ||||
| Field | 8.6 | 75 | 44 | Surface broadcast | [56] |
| 200 | 48 | ||||
| Greenhouse | 8.0 | 55 | 58 | Surface broadcast | [57] |
| 110 | 58 | ||||
| 275 | 61 | ||||
| 550 | 67 | ||||
| 1100 | 63 | ||||
| 2200 | 61 | ||||
| Greenhouse | 8.0 | 55 | 55 | Surface broadcast | [57] |
| 165 | 67 | ||||
| Greenhouse | 8.0 | 55 | 68 | Surface banded |
[57] |
| 165 | 66 | ||||
| Greenhouse | 7.6 | 25 | 22 | Surface broadcast | [58] |
| 50 | 21 | ||||
| 100 | 22 | ||||
| Field | 7.2 | 135 | 15 | Surface broadcast | [59] |
| 225 | 19 | ||||
| Field | 6.3 | 40 | 11 | Surface broadcast (moist) |
[60] |
| 80 | 9 | ||||
| 120 | 8 | ||||
| Field | 6.1 | 30 | 10 | Surface broadcast | [61] |
| 100 | 17 | ||||
| 300 | 33 | ||||
| Laboratory | 6.1 | 50 | 13 | Surface broadcast | [52] |
| 100 | 14 | ||||
| 150 | 16 | ||||
| 200 | 17 | ||||
| 300 | 16 | ||||
| Field | 5.9 | 70 | 24 | Sub-surface Banded (2 cm) |
[53] |
| 140 | 20 | ||||
| 250 | 15 | ||||
| Field | 5.2 | 80 | 14 | Surface broadcast | [54] |
| 160 | 18 |
Table 4: Impact of urea application rate on ammonia losses [53].
Manure type and characteristics such as total nitrogen (TN), ammonium nitrogen (NH4-N) and percentage dry matter (%DM) play an important role in the ammonia volatilization during manure application. Liquid manure (slurry) used for agricultural purposes have high rate of ammonia loss when compared to soil manure. Slurries with lower solids have greater fluidity and can infiltirate soil more readily, where ammonium is protected from volatilization by adsorption onto soil colloids [49,66]. Sommer et al. [66] reported that the composition of animal maure varies widely between animal species and manure types. The animal manure contains a mixture of faeces, urine, blood, split feed, split drinking water and water used in washing and it is collected as slurries. In some animal housing systems, the soild manure containing only faeces and straw and liquid manure containing urine, water and dissolvable faecal components are separated. The typical composition of different types of animal manure are shown in Table 5 [63].
| Manure | Animal | Dry matter (g/Kg) | N. tot (g/Kg) | TAN (g/Kg) | Ureic acid-N (g/Kg) | P (g/Kg) | K (g/Kg) | pH |
| Slurry | Cattle | 74.23 | 3.95 | 1.63 | 0.63 | 3.46 | 7.20 | |
| Pig | 34.50 | 9.35 | 3.66 | 0.74 | 3.62 | 6.72 | ||
| Poultry | 218.00 | 12.00 | 5.93 | 7.23 | ||||
| Solid Manure | Cattle | 181.50 | 4.85 | 1.33 | 1.45 | 3.85 | 7.80 | |
| Pig | 222.00 | 10.45 | 4.40 | 3.70 | 5.25 | 7.70 | ||
| Poultry | 574.60 | 29.60 | 5.49 | 6.0 | 5.98 | 6.53 | 8.50 | |
| Deep Litter | Cattle | 261.00 | 5.20 | 0.90 | 1.40 | 9.70 | 8.60 | |
| Pig | 412.00 | 11.20 | 2.80 | 8.90 | ||||
| Poultry | 570.00 | 27.10 | 6.48 | 7.54 | 9.25 | 15.50 | 9.1 | |
| Liquid Manure | Cattle | 1.68 | 2.60 | 2.05 | 0.03 | 4.33 | 8.70 |
Table 5: Typical composition of animal manure [66].
Ammonia volatilization increases with increases in temperature because at higher temperature the solubiliy of ammonia (NH3) gas is reduced in soil by increasing the proportion of total ammonia nitrogen (TAN) as NH3 gas [67,68]. Sommer et al. [66] reported that TAN is produced by the hydrolysis of urea by ureic acid. Therefore, the ammonia (NH3) emissions from the polutry manure is influenced by water content and temperature. Sommer et al. [65] reported that there is a linear relationship between ammonia volatilization rate and temperature in the range of 15-25°C. Agrifacts [51] reported that 50% of the total nitrogen is volatilized as ammonia at 30°C compared to 25% at 25°C. Therefore, manure applications should not be carried out at higher temperatures.
Thompson et al. [69] reported that wind speed had a positive effect on ammonia (NH3) volatilization but less significant compared to other factors affecting the loss of nitrogen. Increasing the wind speed from 0.5 to 3.0 m/s increased the total 5 day loss by 29% and the loss of nitrogen was more pronounced in the first 24 h after application of manure. Meisinger et al. [52] reported a linear relantionship between ammonia volatilization and wind speeds to about 2.5 m/s.
Application of slury on the soil plays an important role in the ammonia volatilization. There are four types of application techniques (Figure 5) [67]: (a) surface broadcast, (b) surface band-spreading (via trailing hoses), (c) surface placement (via trailing shoes) and (d) shallow slot injection (500 mm depth). During surface broadcaset application the slurry is applied by tanker with a single outlet and splash plate. The surface band spreading involves multiple hoses depositing slury bands 5-10 cm wide on the ground with approximately 30 cm between each bands. The surface placement technique involves placing slurry in bands approximately 3 cm wide and 20 cm apart, between and below the crop canopy. The shallow injection techniquee involves the injected beneath the soil surface either via open slot, shallow injection (to 50 mm) or deep tines (to>150 mm). Misselbrook et al. [70], Smith et al. [71], Huijsmans et al. [72] and Bussink et al. [73] observed direct loss of nitogen fertilizer in the form of ammonia during manure application on soil surface which could be prevented by carrying out shallow injection technique or band spreader technique. Bussink et al. [73] stated that there are other factors such as moisture content, rainfall, soil texture and cation exhange capacity of the soil that affect the volatilization of ammonia druing manure application. The range of measured losses of ammonia at different steps is shown in Table 6 [73].
| Compartment | Ammonia loss | Factors affecting NH3 loss |
| Artificial fertilizer | 6-42% of urea N applied | T, SWC, CEC, pH, fertilizer type, N input, CaCO3, vegetation |
| Application slurry | 1-100% of NH4-N applied on grassland 3-70% NH4-N applied on arable land |
T, rainfall, CEC, SWC, pH, %DM, amount applied, application technique, vegetation |
| Farm yard manure | 45-100% of NH4-N applied | T, CEC, SWC, pH, %DM, amount applied, application technique, vegetation |
| Grazing | 0-18% of the N excreted per grazing or 3.1-8.5% of the N excreted per year |
T, CEC, SWC, N input grassland management, urease activity, rainfall |
| Housing slurry | 0-70% of N excreted | T, stable type, N content of urine, residence time, ventilation |
| Storage of slurry | 0-20% of total N | Time, T, N content, storage type, aeration |
| Crops | <0-156 g N ha-1 d-1 | N status, crop type |
Table 6: Range of ammonia (NH3) losses [73].
Leaching of NO2 and NO3
Leaching of nitrite or nitrate refers to the removal of nitrite or nitrate from the plant root zone by the movement of water through the soil. Since nitrite (NO2-) and nitrate (NO3-) are negatively charged, they are found to move freely with the water unless soils have a significant anion exchange capacity. It was estimated that 55Tg of nitrate are leached from agricultural soils every year [74]. Leaching of nitrogen form soil reduces the bioavailability to plants and impacts the environmental quality.
Ammonium fixation
Soils are found to have the ability to bind ammonium (NH4+) in such a way that it would not be readily recovered by extraction with alkali or dilute acids. This form of ammonium is referred to as nonexchangeable or fixed and is held in between the lattice of clays. It is generally not affected by cations on the clay surfaces or by addition of KCl [75]. However, if ammonium gets exposed due to clay expansion, the exposed ammonium is made available for plant growth [76].
Nitrogen exists naturally in our environment and is constantly being converted from organic to an inorganic form and vice versa. Production of fertilizer adds up to the natural source of nitrogen [77]. Naturally occurring and anthropogenic production of nitrogen make up the whole nitrogen cycle today. Human activities are the biggest contributor of nitrogen and have a major influence on the nitrogen cycle nowadays. The changes in the cycle can affect other natural cycle such as carbon, sulfur and oxygen cycles. Global nitrous oxide emissions are mainly caused by agricultural activities and large scale biomass burning. Agricultural emissions show a strong increase due to the application of fertilizer to agricultural soils and due to grazing of animals and spreading of animal manure. The inter annual variability of agricultural N2O emission is caused by the annual savannah burning. Table 7 summarize the global N2O emissions from number of resources around the world [78]. Table 8 shows the N2O emission by source in Canada in 2010 [79]. Overall, approximately 140TgNy-1 was fixed by human activity in terrestrial ecosystem [9]. The sources and pathways of nitrogen that result in direct and indirect N2O emissions from soils and water are shown in Figure 6 [80].
| Source | 1992 | 1995 | 1997 | 2002 | 2008 |
| Manure management | 317.81 | 320.40 | 304.14 | 313.00 | 336.00 |
| Direct soil emissions | 2231.80 | 2296.46 | 2370.49 | 2480.00 | 2690.00 |
| Manure in pasture/range/paddock | 1672.96 | 1727.17 | 1731.01 | 1810.00 | 1980.00 |
| Indirect N2O emission from agriculture | 746.26 | 767.57 | 780.44 | 819.00 | 894.00 |
| Savanna burning | 897.03 | 629.10 | 582.99 | 503.00 | 907.00 |
| Agricultural waste burning | 31.81 | 32.45 | 33.86 | 35.30 | 40.90 |
| Forest fires | 424.66 | 287.48 | 305.94 | 285.00 | 185.00 |
| Grassland fires | 97.92 | 96.32 | 87.02 | 117.00 | 23.80 |
| Peat fires and decay of drained peatland | 85.28 | 17.26 | 310.67 | 70.70 | 2.22 |
| Forest Fires-Post burn decay | 275.18 | 290.19 | 285.81 | 292.00 | 276.00 |
| Wastewater handling | 268.56 | 285.83 | 297.43 | 320.00 | 342.00 |
| Waste incineration | 6.16 | 6.36 | 6.49 | 6.77 | 7.39 |
| Other waste handling | 8.49 | 11.74 | 13.83 | 19.10 | 25.70 |
| Fossil fuel fires | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| Indirect N2O from non-agricultural NOx | 536.54 | 520.04 | 536.71 | 536.00 | 603.00 |
| Indirect N2O from non-agricultural NH3 | 273.97 | 137.00 | 558.50 | 224.00 | 117.00 |
| Total | 7875.18 | 7426.12 | 8206.09 | 7831.62 | 8430.76 |
Table 7: Global N2O emissions in Tg N2O [78].
| Source | Nitrous Oxides(Kilotonnes) |
| Transportation | 680.0 |
| Off-road vehicles | 458.1 |
| Oil and Gas industries | 457.5 |
| Fuel for electricity and heating | 277.5 |
| Other industries | 174.6 |
| Home firewood burning | 10.1 |
| Incineration and miscellaneous | 2.4 |
Figure 6: Sources of nitrogen that results N2O emissions [80].
Atmospheric precipitation
Most of the nitrogen in the atmosphere is in the molecular form of N2. However, there are small amounts of ammonia (NH3) as well as various nitrogen oxides (NO2), such as nitric oxide (NO) and nitrous oxide (N2O) and their hydration products nitric acid (HNO3), present in the atmosphere [81]. Most atmospheric nitrogenous compounds are attributed to industrial air pollution. Small amounts are released from the decomposition of organic matter in the soil and photochemical reactions in the atmosphere [80,82]. Most of dissolved inorganic N in rain water is ammoniac nitrogen. Cape et al. [83,84] reported that organic nitrogen makes up 24-40% of the total amount of dissolved nitrogen in rain and snow across UK (Table 9). Maximum concentration of ammonium and nitrate occur during spring while late summer rain contains more water-soluble organic nitrogen. Goldberg [85] stated contribution from rainwater to nitrogen added to soil or runoff water vary greatly depending upon time of the year and the location which have great implication on the amount of fertilizer used by farmers and the consequent air and water pollution.
| Site | Nitrate-N | Ammonium-N | Organic-N | Total | |||
| (μM) | (%) | (μM) | (%) | (μM) | (%) | (μM) | |
| Cairngorm | |||||||
| North-east Scotland | 30 | 52 | 14 | 24 | 14 | 24 | 58 |
| Bush | |||||||
| East Scotland | 15 | 33 | 16 | 35 | 15 | 33 | 46 |
| Merlewood | |||||||
| North-west England | 17 | 19 | 27 | 31 | 35 | 40 | 87 |
| Moor house | |||||||
| North-west England | 20 | 24 | 32 | 38 | 31 | 37 | 84 |
| Climoor | |||||||
| North Wales | 22 | 30 | 33 | 45 | 29 | 39 | 73 |
| Norwich University | |||||||
| South-East England | 38 | 29 | 58 | 45 | 3 | 26 | 129 |
| Winfrith | |||||||
| South-west England | 23 | 31 | 26 | 35 | 23 | 31 | 75 |
Table 9: Concentration (μM) of inorganic and total nitrogen in precipitation across United Kingdom during the period of 2000-2002 [84].
Production of nitrous oxide (N2O) is one of the greatest concerns today as it is one of the main greenhouse gases. Earlier estimation of the global budget of N2O production, suggested that the emission of N2O to the atmosphere by agricultural activities was relatively small [86-89]. However, recent investigation suggested that agricultural activities can actually contribute a lot more N2O emission than was thought earlier [83,84]. If total agricultural emission is to be considered, N2O emission can be produced not only from fields fertilized with synthetic nitrogen fertilizer but also includes animal production system [90]. Volatilization, runoff and leaching are some of the mechanisms through which nitrogen is being release back to the environment from agricultural activities. However, with human population exceeding seven billion people in 2012, the nitrogen emission from agricultural activities needs a further investigation [91]. As human population continues to increase rapidly, it is only a matter of time that nitrogen pollution from agricultural production systems will be our biggest concern of all.
The most abundant oxide of nitrogen is probably nitrogen tetroxide (N2O4), produced by internal combustion engines. More than 20 Tg of N is fixed or mobilized during fossil fuel combustion and other high temperature process which is emitted in a form of NO. Ion molecule reactions, which occur in the stratosphere and upper ionosphere, account for formation of nitrogen molecules other than N2 [92].
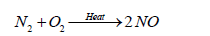 (18)
(18)
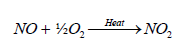 (19)
(19)
The formation of ammonia in the atmosphere is mainly due to the effect of lighting. Some of this ammonia is oxidized to some oxides of nitrogen. Butcher et al. [93] reported that formation of two molecules of NO from collision of N2 and O2 in a direct heat process is relatively slow reaction compared to the formation of NO in the presence of lightening which results in a series of simple steps comprising of Zeldovich mechanism as shown in the following reaction:
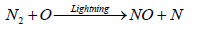 (20)
(20)
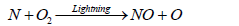 (21)
(21)
Geological sources
A very good example of geological sources of nitrate is the sediments of nitrate of soda on the plateau of Tarapacá, a part of Atacama Desert in Chile. The nitrate deposits in Chile have probably been developed since the ancient times. Evans et al. [94], Noble [95] and Ehleringer et al. [96] stated that Atacama Desert had to go through decades of intervals of rainfall. The almost no-existence moisture in that area causes Atacama Desert to be known as the driest places on Earth. Since nitrate is soluble in water, the absence of water allow nitrate to deposits in layers forming caliche, type of sedimentary rock that consists layers of sodium nitrate. Holloway et al. [97] and Blatt et al. [98] reported that 75% of rocks exposed at the Earth’s surface are sedimentary in nature containing 1021 g of fixed nitrogen globally which is a lot more than the amount of fixed nitrogen in the total biosphere of 1019 g. The formation of nitrogen in the bedrocks was derived from burial of organic matter in marine and freshwater sediments [86].
Other sources of nitrate are igneous rocks, metamorphic rocks and coal. These fixed forms of nitrogen in rocks may amount in some estimation to a total 50 times greater than the amount of fixed nitrogen in the atmosphere. Although rocks contain significant amount of fixed nitrogen, it was not thought as a ready source due to access problem [97,99]. Morford [99] suggested that nitrogen rich bedrocks may influence the forest ecosystem ability to store carbon and nitrogen. Bedrocks rich in nitrogen might have been feeding the forest, enhancing its growth. Nitrogen content of soils and forest foliage on nitrogen rich sedimentary rocks is 50% more than the soil and forest foliage on nitrogen poor bedrocks. Forest associated with nitrogen rich bedrocks also have 42% more carbon in above ground tree biomass and 60% more carbon in the upper 30 cm of the soil in comparison to similar sites on top of poor nitrogen bedrocks. These findings may explain why the nitrogen budget for forest is always imbalanced.
The role of nitrogen as a nonpoint sources to the contamination of surface water was reported by Holloway et al. [97] who observed increased nitrogen content in stream water in certain California watersheds caused by bedrock lithology. The Mokelumne River watershed in the central Sierra Nevada of California experienced consistently low nitrogen concentration in its stream water at its lower watershed that has diorite lithology. While for other streams that have biotite schist lithology, the concentration of nitrogen in the streamwater was highest during the rainy season and decreasing during the spring period before the end of the stream flow. Lower watershed that contained geological nitrogen was the source of 90% of nitrogen in the streamwater in comparison to upper watershed. Although upper watershed had 90% of watershed area, the nitrogen flux was only 0.12 kg N ha-1 yr-1 while the lower watershed had nitrogen flux of 10-20 kg N ha-1 yr-1 during the 1995-96 water year. Significance difference between the two parts of watershed suggested that geological nitrogen would be the only source.
Agricultural land
Agricultural sources of nitrogen result primarily from organic and inorganic materials added to soils as a source of crop nutrition. Since fertilizers are now one of the lowest-cost “inputs” in crop production, the use of synthetic nitrogen is growing rapidly in areas where natural soil phosphorus, potassium and calcium are inadequate and limit growth [100]. Kjaergaard [101] reported that synthetic nitrogen was added to the soil in Europe when the area of fallow land was decreasing, limiting the available nitrogen for the crops.
Increasing world population causes more than ever a demand for food and boosts up the production of synthetic nitrogen for agricultural land. Galloway [102] stated that for food production per capita basis to increase, additional nitrogen must be added to agro-ecosystem to keep up with the demand. Supply and demand of fertilizer in the world has steadily increased since 2007 and the trend is predicted to be the same for the next fifty years [93]. The supply and demand balance for the nitrogen has also increasing (Table 10) [103]. The total world nitrogen fertilizer supply increased form 131.106 Tg/year in 2007 to 154.199 Tg/ year in 2012.
| Nitrogen | 2008 | 2009 | 2010 | 2011 | 2012 | |||||
| Fertilizer | Nitrogen | Fertilizer | Nitrogen | Fertilizer | Nitrogen | Fertilizer | Nitrogen | Fertilizer | Nitrogen | |
| Total supply | 206431 | 131106 | 212225 | 136252 | 219930 | 140732 | 230334 | 147748 | 240711 | 154199 |
| Total demand | 197004 | 127820 | 201482 | 130409 | 205947 | 133059 | 211230 | 136198 | 216019 | 139140 |
| Surplus | 9427 | 3286 | 10473 | 5843 | 13983 | 7673 | 19104 | 44550 | 24692 | 15059 |
Table 10: Supply and demand of fertilizer and nitrogen (thousand tonnes) in the world for the duration of 2008-2012 [103].
Table 11 shows the top ten countries with highest fertilizers application per a hectare of land [103]. China, one of the largest agricultural countries in the world, increased the use of chemical fertilizer from 1.74 × 107 Mg N in 1990 to 2.2 × 107 Mg N in 1995 and used 54 million tonnes of fertilizers in 2011 [105]. The fertilizers used in China alone accounted for one fourth of the world total fertilizers [103-106]. The rapid increase in fertilizer use in China is polluting ground water and surface water and causing many environmental and health problems. Besides synthetic fertilizer, China still uses animal manure and human faeces as organic fertilizers.
| Country | Amount of fertilizers used (Kg/ha) |
| Ireland | 594.5 |
| Netherlands | 450.2 |
| Egypt | 385.8 |
| Costa Rica | 385.0 |
| Slovenia | 369.4 |
| Japan | 301.0 |
| United Kingdom | 285.8 |
| Vietnam | 285.3 |
| Israel | 256.0 |
| China | 255.6 |
Table 11: List of countries with the highest consumption of fertilizers in the world [104].
In the 17th century, the animal manure collected from animal in grazing season and the biological nitrogen fixation were enough to provide about 20 Kg N ha-1 for grain production of 1999 kg ha-1 in Western Europe [107]. Currently, synthetic fertilizers have been used to provide much higher crop yield. Artificial fertilizers are widely used to provide three types of primary plant nutrients: nitrogen (N), phosphate (P2O5) and potash (K2O). Table 12 shows the use of fertilizer in USA from 2007 to 2010 for agricultural activities [108].
| Year | 1000 tonnes | |||
| Nitrogen | Phosphate (P2O5) | Potash (K2O) | Total | |
| 2007 | 13194 | 4572 | 5133 | 22899 |
| 2008 | 12561 | 4247 | 4660 | 21468 |
| 2009 | 11461 | 3138 | 3090 | 17689 |
| 2010 | 12285 | 4099 | 4458 | 20843 |
Table 12: Fertilizer use in USA for the years of 2007-2010 [106].
a
Different forms of added nitrogen as fertilizers could be lost from the soil to the surface water and underground water causing pollution problems. The nitrogen salts (NO2 and NO3) carried by the runoff are in a direct relation to the amount of water applied and land use practice. Drainage water contains nitrogen concentrations of 1-60 mg/L, mostly in the form of nitrate [109]. Sediment suspended in the flowing water may carry relatively high amounts of ammonium nitrogen as well as particulate organic nitrogen [110-112].
The lateral and vertical movements of nitrogen in the soil are quite different in their effect as a source of pollution. Iqbal [113] indicated that the vertical movement of nitrogen is more significant than the lateral movement as a source of pollution. Nitrate dissolves easily in water and moves through soil pores, contaminating ground water as shown in Table 13 [113]. Nitrate horizontal potential movement in soils is negligible and can be negative in shallow subsoil. Platzer [114] stated that the vertical flow has a very high nitrogen capacity while the horizontal flow associates effectively with denitrification.
| Fertilizer application kg ha | Nitrate vertical leaching (mg) | Nitrate lateral leaching | ||||||||
| 30 cm | 60 cm | Amount (mg/L) | Movement (mg/m) | |||||||
| I | F | S | T | I | F | S | T | |||
| 0 | 50.13 | 134.74 | 43.95 | 101.13 | 57.12 | 75.39 | 43.95 | 123.63 | 5.79 | 1.22 × 10-3 |
| 90 | 102.62 | 58.87 | - | 128.24 | 72.60 | 119.54 | 47.84 | 143.24 | 8.97 | 3.24×10-3 |
| 180 | 142.23 | 102.49 | - | 185.17 | 112.47 | 61.49 | 85.27 | 147.31 | 5.06 | -1.50×10-3 |
| 270 | 129.00 | 125.73 | 92.38 | 209.35 | 63.57 | 89.17 | 77.88 | 212.39 | 13.43 | 7.58×10-3 |
| 360 | 230.28 | 83.11 | 88.43 | 196.71 | 146.37 | 43.88 | 88.43 | 214.89 | 4.92 | -1.06×10-3 |
Table 13: Vertical and lateral nitrate leaching in a wheat farm [113].
Subsurface drainage of gravitational water from the soil through tiles is commonly used in the agriculture to improve crop production in poorly drained soils. The drainage water from the crops contains significant amounts of nitrate-N (NO3-N) [115]. Bolton [116] conducted a tile drainage experiment at Woodslee, Ontario, Canada on a Brookston clay soil and estimated the nutrient losses through tile drains under three different cropping systems mainly in the nitrate form with and without addition of fertilizers. They found that the greatest losses of nitrogen occurred with corn grown continuously or in rotation. They reported that the losses were increased by fertilizer application and were associated with the total and average effluent flows as shown in Table 14 [116].
| Crop | N Losses (Kg/ha/y) | Effluent from drains (ppm) | ||
| No Fertilizer | Fertilizer | No Fertilizer | Fertilizer | |
| Rotation | ||||
| Corn | 5.6 | 15.1 | 8.5 | 14 |
| Oats and alfalfa | 4.3 | 5.7 | 6.4 | 8.5 |
| Alfalfa (1st year). | 4.8 | 3.9 | 6.3 | 5.8 |
| Alfalfa (2nd year) | 4.7 | 8.6 | 9.3 | 10.1 |
| Continuous | ||||
| Corn | 6.6 | 14 | 4.4 | 8.9 |
| Bluegrass sod | 0.3 | 0.7 | 3.5 | 1.1 |
| Mean | 4.4 | 8.1 | 6.4 | 8.1 |
Table 14: Nitrogen annual losses through tile drains and concentration in effluent of tile drains on Brookston clay soil [116].
Randall et al. [117] studied the nitrate losses through subsurface tile drainage in four cropping systems including: continuous corn, a corn-soybean rotation, alfalfa and conservation reserve program. The conservation reserve program (CRP) was carried out in United States of America as part of converting highly erodible and environmentally sensitive agricultural land to permanent grassland cover to reduce soil erosion and produce row crops [118]. The results from the study indicated that average NO3-N concentrations from continuous corn, corn-soy bean rotation, alfalfa and conservation reserve program (CRP) were 32, 24, 3 and 2 mg/L, respectively. Nitrate losses from continuous corn and corn-soybean were 37 times and 35 times more than alfalfa and CRP systems, respectively.
Kladivko et al. [119] summarises the results from the study conducted over 15 years on nitrate leaching to subsurface drains. The results indicated that the mean nitrate concentration decreased from 28 mg/L in the period of 1986-1988 to 8 mg/L in the period of 1997-1999. The results also showed that the annual nitrate leaching decreased from 38 kg/ha in the period of 1986-1988 to 15 kg/ha in the period of 1997-1999, respectively. The reduction in the concentration of nitrate loss was due to reduction in the fertilizer N rates and addition of a winter cover crop as a trap crop after corn-soybean rotation. The author concluded that nitrate concentrations and nitrate losses in the tile drains vary with soil organic matter, yearly weather conditions, fertilizer N rates and timing, drain spacing, cover crop growth, cash crop yield and water table control practices.
Randall and Goss [120] reported that some non-controllable factors such as precipitation and mineralization of soil have great impact on the drainage volume and nitrate loads which put limits on the concentrations and loads that can be achieved by row-crop agriculture.
Livestock and poultry operations
In 2009, Luxembourg recorded the highest meat consumption per capita (194.2 kg/year) followed by U.S (190.6 kg/year). Canada was in the 16th place with meat consumption per capita of 152 kg/year. The top 20 meat consumption countries are shown in Table 15 [121]. Currently, chicken is the highest available stocks in the world, followed by cattle and pigs as shown in Table 16 [122]. Today, China leads the total meat consumption in the worl by consuming 71 million tonnes of meat per year. Half of the pork in the world is in China. China’s pork consumption rose to 52 million tonnes in 2012 [123]. Canada has 12.5 million cattle on their farms in 2012. The beef cows were 4.2 million, the beef replacement heifers were 554,300, the dairy cows were 1.4 million and the cattle and calf slaughter totalled 3.5 million [124]. The Canadian cattle inventories from 2006-2012 are shown in Table 17.
| Rank | Country | Per Captia (kg/yr) |
| 1 | Luxembourg | 194.2 |
| 2 | United States of America | 190.6 |
| 3 | Austria | 185.1 |
| 4 | Australia | 183.4 |
| 5 | New Zealand | 179.0 |
| 6 | Oceania | 173.4 |
| 7 | Denmark | 170.6 |
| 8 | Spain | 163.0 |
| 9 | Argentina | 161.8 |
| 10 | Bahamas | 160.3 |
| 11 | Italy | 158.4 |
| 12 | Portugal | 157.0 |
| 13 | Bermuda | 156.8 |
| 14 | Germany | 156.3 |
| 15 | French Polynesia | 155.4 |
| 16 | Canada | 152.0 |
| 17 | Mongolia | 151.1 |
| 18 | Slovenia | 149.6 |
| 19 | Ireland | 147.8 |
| 20 | France | 146.5 |
Table 15: Meat consumption per captia of top 20 countries in 2009 [121].
| Animal | Number in××104 |
| Asses | 4,323.0700 |
| Beehives | 7,841.1600 |
| Buffaloes | 19,539.7500 |
| Camelids, other | 839.1900 |
| Camels | 2,663.5400 |
| Cattle | 1,42,638.9000 |
| Chickens | 2,070.8000 |
| Ducks | 132.38500 |
| Geese and guinea fowls | 38.1200 |
| Goats | 92,414.5900 |
| Horses | 5,847.2100 |
| Mules | 1,045.7100 |
| Pigeons, other birds | 3.2500 |
| Pigs | 96,716.4600 |
| Rabbits and hares | 89.5000 |
| Rodents, other | 1.8400 |
| Sheep | 1,09,356.6700 |
| Turkeys | 46.7600 |
Table 16: World stocks of live animals in 2011 [122].
| Cattle | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
| Bulls | 264.0 | 244.6 | 246.8 | 243.9 | 232.1 | 222.2 | 221.2 |
| Beef cows | 5,247.2 | 5,020.1 | 4,981.9 | 4,649.5 | 4,391.0 | 4,273.0 | 4,228.4 |
| Dairy cows | 1,019.1 | 994.8 | 984.3 | 978.5 | 981.0 | 983.1 | 985.3 |
| Heifers, dairy | 495.1 | 480.1 | 471.1 | 450.6 | 450.7 | 443.1 | 444.1 |
| Heifers, beef | 628.3 | 587.1 | 595.0 | 537.0 | 516.4 | 531.6 | 554.3 |
| Heifers, slaughter | 986.8 | 963.5 | 982.9 | 834.5 | 899.8 | 844.0 | 839.5 |
| Steers | 1,146.8 | 1,145.2 | 1,101.6 | 1,067.6 | 1,141.7 | 1,081.7 | 1,098.1 |
| Calves | 4,867.7 | 4,719.6 | 4,506.4 | 4,433.4 | 4,292.3 | 4,078.3 | 4,144.1 |
| Total | 14,655.0 | 14,155.0 | 13,870.0 | 13,195.0 | 12,905.0 | 12,457.0 | 12,515.0 |
Table 17: Cattle inventories of Canada from 2006-2012 [124].
Animals are not breed solely for the meat but also for milk, eggs and fibers. The high demand for meat based foods changed the way food is produced today [125,126].
The system known as concentrated animal feeding operation (CAFO) allows for growing thousands of animals on strict diets. Phang [127] reported that animal feeding operation is an agricultural operation where animals are kept and raised in confined areas. Feed is bought to the animals rather than the animals grazing for their feed. A farm reaches the status of CAFO when it houses at least 300 cattle, 750 pigs or 25,000 chickens. In the CAFO system, animals are fed with high nutrients diet including nitrogen rich foods.
Olson et al. [128] reported that increasing meat consumption around the world justifies the needs of having CAFO as this system provide the meat needed in large amount with lowest cost. There are several advantages of CAFOs including: (a) low-cost source of meat, milk and eggs, (b) efficient feeding and housing of animals, (c) increased facility size and (d) animal specialization. There are also major environmental risks involved in this method of housing animals. The most health related issue is the production of large amounts of manure which contains plant nutrients such as nitrogen and phosphorous, pathogens such as E. coli, growth hormones, anitbiotics, chemical additives used to clean manure, animal blood, leachate from corn feed and copper sulfate used in the footbath for cows [13,125,129,130 ].
Burkholder et al. [131] stated that growing the animal in CAFO systems is unnatural and unhealthy. The animals are confined in a very limited space where they cannot even turn around. They are fed with enormous amount of corn and soy beans to increase their weight significantly in the shortest time. High density, limited space and continuous supply of food will produce large volume of manure. Sherman [132] stated that manure confined feeding operations is heavier because the manure is a mixture of faeces and urine with additional water used to pump it to the storage facility. The heavy manure is hard to be transported and disposing it has caused a major form of concern because of its impact to the environment).
Table 18 shows the number of farm animals slaughtered for human consumption in the world [133]. Table 19 shows the amount of manure produced by different animals [134-136]. USDA [137] estimated the manure production to be more than 335 million tonnes/year on the farms in United States, which was 100 times more than the manure produced by humans. It is estimated that the amount of manure produced by 2,500 cows are equal to the waste produced by 411,000 humans (1 cow=164.4 persons).
| Animals | Heads |
| Buffalo | 57,681,750.00 |
| Cattle | 609,729,971.76 |
| Ass | 2,461,800.00 |
| Camel | 2,031,008.00 |
| Goat | 879,124,344.00 |
| Horse | 4,504,308.00 |
| Mule | 542,600.00 |
| Camelids | 655,712.00 |
| Pig | 1,382,927,239.95 |
| Sheep | 1,046,814,221.65 |
Table 18: Total number of farmed animals slaughtered for human consumption in 2011 [133].
| Animal | Average body weight (kg) | Urinary N (g/d) | Fecal N (g/d) | Total N (g/d) |
| Cattle | 400 | 90.0 | 70.0 | 160.0 |
| Sheep | 40 | 7.0 | 3.0 | 10.0 |
| Goats | 15 | 5.0 | 3.0 | 8.0 |
| Horses | 400 | 49.0 | 27.0 | 76.0 |
| Pigs | 80 | 15.0 | 3.0 | 18.0 |
| Chicken | 4 | 7.2 | 3.4 | 10.6 |
Table 19: Nitrogen content in animal excretion [134-136].
Manure from concentrated animal feeding operations is usually collected in a lagoon near the feedlots. The manure is disposed either by pumping the liquefied manure onto nearby “sprayfields” (crops, pasture or forage fields fertilized and irrigated by nutrient rich liquid) or being transported to another location with a high cost of transportation [138,139]. These practises increase the amount of nitrogen released freely into the environment which results in air, soil and water pollution. However, in recent years, the economic advantages of commercial fertilizers have made the use of animal wastes unprofitable to many farmers. The cost of handling and treating manure reduced the popularity of using it as organic fertilizer. The large automated feeding operations further magnified the problem by concentrating thousands of animals in localized areas [125].
Air pollution is mainly caused by various emissions from CAFOs such as ammonia, hydrogen sulfide, volatile organic acids, methane and particulate matter. The type of air pollutants surrounding CAFOs are shown in Table 20 [125]. Varel [140] reported on the nitrogen loss from feedlots via volatilization of nitrogen gases, primarily ammonia, into the atmosphere. Ammonia volatilization has contributed to soil and water pollution, where 90% of the nitrogen in urine exerted in cattle feed yards may escape into the air as ammonia. The authors found that a significant amount of ammonia volatilized from cattle feedlots is returned from the air to water bodies in the vicinity of the feedlot, and so the magnitude of nitrogen enrichment of lakes via this pathway can be significant.
| CAFO emissions | Source | Traits | Health risks |
| Ammonia | Formed when microbes decompose undigested organic nitrogen compounds in manure | Colorless, sharp pungent odor | Respiratory irritant, chemical burns to the respiratory tract, skin and eyes, severe cough, chronic lung disease |
| Hydrogen Sulfide | Anaerobic bacterial decomposition of proteins and other sulfur containing organic matter | Odor of rotten eggs | Inflammation of the moist membranes of eye and respiratory tract, olfactory neuron loss, death |
| Methane | Microbial degradation of organic matter under anaerobic conditions | Colorless, odorless, highly flammable | No health risks. Is a greenhouse gas and contributes to climate change |
| Particulate matter | Feed, bedding materials, dry manure, unpaved soil surfaces, animal dander, poultry feathers | Comprised of fecal matter, feed materials, pollen, bacteria, fungi, skin cells, silicates | Chronic bronchitis, chronicrespiratory symptoms, declines in lung function, organic dust toxic syndrome |
Table 20: Air Pollutants from CAFO emissions [125].
Ndegwa et al. [141] reported that ammonia volatilization is one of the major pathways for nitrogen loss from agricultural feeding operations. This process of losing nitrogen not only affects agricultural production but can adversely affect the environment. Ammonia deposited in the atmosphere in lower amounts are beneficial to plants as a nutrient source for growth but when excess N is deposited, it gets oxidized and reduced and causes various environmental issues including: (a) exposure to high concentrations of fine particulate aerosols (PM2.5) causes respiratory diseases, (b) contamination of drinking water with nitrate, (c) eutrophication of surface water bodies causing algal blooms and decreased water quality; (d) higher concentrations of N causes changes in vegetation and ecosystem, (e) climatic changes associated with increases in nitrous oxide (N2O), (f) saturation of forest soils with nitrogen and (g) acidification of soil through nitrification and leaching.
Water pollution results mainly due to the insufficient land used to contain the volume of manure. Runoff and leaching from feedlots have always been a threat to the nearby water bodies but a lagoon failure can send tens of millions of liters of untreated manure directly into water bodies. Minimal leaching and accumulation of nitrogen below the feedlot pens can possibly occur from denitrification of the poorly aerated layers [131,142]. In a study of soil and groundwater quality under a cattle feedlot in Southern Alberta, Olson et al. [128] reported that denitrification may have prevented an increase in NO3-N concentration in the groundwater. However, concentration of NH4-N increased in the shallow groundwater beneath the pens although it did not accumulate in the sub soil layer during the four years of study.
Westerman et al. [138] evaluated swine lagoon seepage in sandy soils. They investigated two swine manure anaerobic lagoons on coastal sandy soil. Both lagoons had significant seepage after 3.5-5 years. The authors reported that manure lagoons constructed in sandy soils with high water tables have resulted in some groundwater pollution and about 3-6 mg nitrate NO3/L were found in surface runoffs from spray fields. They concluded that too much variability of NH3-N and NO3-N with time which made it difficult to determine the factors affecting the pollutant transport.
Stone et al. [139] conducted a 5 year research study on a watershed stream with intensive agricultural practices of crop, swine, poultry and cattle production. The results from the study indicated that most of the streams in the watershed had acceptable water quality. The nitrogen mass balance calculations and comparisons of the N loadings in the streams showed that 26% of the excess nitrogen applied is lost to stream water. The streams adjacent to swine effluent spray fields had 6-8 mg total inorganic N/L and 0.7-1.3 mg P/L.
Mahmoodabadi et al. [143] studied the effect of sheep manure leaching on soybean root characteristics and soil salinity. The results from the study indicated that the sheep manure significantly increased N, Fe, Cu, Zn and Mn concentrations. There was no significant increase in the pH of the soil (from 7.8 to 7.9). The leaching manure helped in increasing the number and dry weight of root nodules. Applying sheep manure increased the net nitrogen fixation in root nodules to 1.81 mg/ Kg compared to 0.54 mg/Kg in the control pot. The sheep manure also reduced the salinity in the soil by 2%.
Dikinya et al. [144] studied the effect of chicken manure on three different soils including: luvic calcisol, ferralic arenosol and vertic luvisol that were classified as sandy clay loam, loamy sand and clay, respectively. The experiment was conducted for 63 days and the results indicated that there was substantial increase in pH. The highest pH recorded was 7.75 for ferralic arenosol soil and the lowest pH recorded was 7.01 for vertic luvisol soil. For most of the time the pH for the soil treated with chicken manure remained neutral or slightly alkaline. Lopez-Mosquera et al. [145] also reported that soil pH treated with chicken manure remained in the range of 7-7.9.
Whalen et al. [146] studied the effect of cattle manure on the pH of two acid soils obtained from Beaverlodge and Fort Vermillion in the Peace River Region of Alberta, Canada. The results obtained from the 8 week study indicated that the pH of Beaverlodge and Fort Vermillion soils increased from 4.8 to 6.0 and 5.5 to 6.3, respectively. The availability of minerals such as N, P, K, Ca and Mg increased immediately after manure application and were 3 to 4 times higher than untreated soils.
Plaza et al. [147] studied the effect of annual additions of pig slurry on soil pH at the rate of 30, 60, 90, 120 and 150 m3h a-1 y-1 over a 4 year period under semiarid conditions. The control soil had a pH of 6.0. After slurry application the pH of soil increased significantly and the highest pH of 7.6 was observed. The soil had greater P and K contents and slightly higher total N content in it. However a significant decrease in the organic C was observed in soil treated with high slurry rates. The effect of animal manure of the pH of the soil from several other studies is shown in Table 21 [148-157].
| Type of manure | Characteristics (gKg-1DM) | Time | pH change | Reference | ||||||
| C | N | P | K | Ca | Na | Mg | ||||
| Poultry(L) | 250.0 | 39.0 | 205.0 | 26.9 | 110.7 | 5.4 | 11.0 | 6 weeks | 4.0→6.5 | [148] |
| Poultry(B) | 343.0 | 25.0 | 188.0 | 19.0 | 34.8 | 3.5 | 17.0 | 6 weeks | 4.0→5.0 | [148] |
| Poultry | 211.5 | 13.7 | 13.0 | 5.2 | 69.5 | 2.0 | 19.5 | 3 weeks | 4.7→5.8 | [149] |
| Poultry | 239.0 | 63.0 | 21.0 | 20.0 | 77.0 | 2.6 | 5.7 | 6 weeks | 4.2→5.4 | [150] |
| Poultry | 252.0 | 39.0 | 20.5 | 26.9 | 110.7 | -- | 11 | 3 days | 4.1→6.3 | [151] |
| Poultry | 131.0 | 32.8 | 43.0 | 29.3 | 27.0 | -- | 5.7 | 30 days | 4.3→4.6 | [152] |
| Poultry | 296.3 | 3.6 | -- | -- | 12.4 | -- | 8.9 | 3 weeks | 4.6→6.4 | [153] |
| Poultry | 252.0 | 38.5 | 20.5 | 26.9 | 110.7 | 5.4 | 11.0 | 7 weeks | 4.1→6.5 | [154] |
| Poultry | 252.0 | 38.5 | 20.5 | 26.9 | 110.7 | 5.4 | 11.0 | 25 weeks | 4.1→5.0 | [154] |
| Cattle | 114.0 | 10.0 | 1.8 | 0.60 | 0.3 | -- | 0.1 | 3 days | 4.1→4.6 | [151] |
| Cattle | 249.3 | 22.8 | 7.0 | 22.7 | -- | -- | -- | 25 weeks | 4.8→6.0 | [155] |
| Cattle | 313.0 | 4.9 | -- | -- | 11.2 | -- | 19.9 | 3 weeks | 4.6→5.9 | [153] |
| Cattle | 194.0 | 16.3 | 3.8 | 4.8 | 2.4 | 0.1 | 0.8 | 7 weeks | 4.1→4.6 | [154] |
| Cattle | 194.0 | 16.3 | 3.8 | 4.8 | 2.4 | 0.1 | 0.8 | 25 weeks | stable | [154] |
| Pig | 340.0 | 25.4 | 34.5 | 12.4 | 50.9 | 4.0 | 11.5 | 7 weeks | 4.1→5.0 | [154] |
| Pig | 340.0 | 25.4 | 34.5 | 12.4 | 50.9 | 4.0 | 11.5 | 25 weeks | 4.1→4.2 | [154] |
| Pig (RL) | -- | 28.8 | 0.66 | 1.1 | -- | -- | -- | 28 days | 5.8→5.3 | [156] |
| Pig (RL) | -- | 28.8 | 0.66 | 1.1 | -- | -- | -- | 120 days | 5.8→5.2 | [156] |
| Pig (ADL) | -- | 30 | 0.78 | 1.1 | -- | -- | -- | 28 days | 5.8→5.3 | [156] |
| Pig (ADL) | -- | 30 | 0.78 | 1.1 | -- | -- | -- | 120 days | 5.8→5.2 | [156] |
| Pig | 271.3 | 5.2 | -- | -- | 13.7 | -- | 13.0 | 3 weeks | 4.6→6.4 | [153] |
| Pig | -- | 24.2 | 39.0 | 16.5 | 38.6 | -- | 9.9 | 8 weeks | 4.0→4.8 | [157] |
Table 21: Effect of animal manure application on soil pH.
Urban waste
Domestic waste water effluents without specific treatment for nitrogen removal have nitrogen concentrations in the range of 20- 85 mg N/L [158,159]. A typical composition of domestic wastewater is shown in Table 22 [160]. Ammonium is the predominant from of nitrogen in effluents from primary and high rate treatment plants. Storm water from residential areas can supply some nitrogen to rivers. Analysis of Wei River in China showed that it contains 7.31 mg NH4/L [161]. Zhang et al. [162] noted down ammonium concentration of 10- 28 mg/L when examined the Yellow River in China.
| Contaminants | Concentration (mg/L) | ||
| Low strength | Medium strength | High strength | |
| Solids, total (TS) | 390 | 720 | 1230 |
| Dissolved, total (TDS) | 270 | 500 | 860 |
| Fixed | 160 | 300 | 520 |
| Volatile | 110 | 200 | 340 |
| Suspended solids, total (TSS) | 120 | 210 | 400 |
| Fixed | 25 | 50 | 85 |
| Volatile | 95 | 160 | 315 |
| Settleable solids | 5 | 10 | 20 |
| Biochemical oxygen demand | |||
| 5-d, 20°C (BOD5, 20°C) | 110 | 190 | 350 |
| Total organic carbon (TOC) | 80 | 140 | 260 |
| Chemical oxygen demand (COD) | 250 | 430 | 800 |
| Nitrogen (total as N) | 20 | 40 | 70 |
| Organic | 8 | 15 | 25 |
| Free ammonia | 12 | 25 | 45 |
| Nitrites | 0 | 0 | 0 |
| Nitrates | 0 | 0 | 0 |
| Phosphorous (total as P) | 4 | 7 | 12 |
| Organic | 1 | 2 | 4 |
| Inorganic | 3 | 5 | 10 |
| Chlorides | 30 | 50 | 90 |
| Sulfate | 20 | 30 | 50 |
| Oil and grease | 50 | 90 | 100 |
| Volatile organic compounds (VOCs) | <100 | 100-400 | >400 |
| Total coliform (no./100mL) | 106-108 | 107-109 | 107-1010 |
| Fecal coliform (no./100mL) | 103-105 | 104-106 | 105-108 |
| Cryptosporidumoocysts(no./100mL) | 10-1-100 | 10-1-101 | 10-1-102 |
| Giardia lambia cysts( no./100mL) | 10-1-101 | 10-1-102 | 10-1-103 |
Table 22: Typical composition of untreated domestic wastewater [160].
Several substances containing nitrogen are commonly found in industrial wastes. Ammonia is a waste material from gas and coke manufacturing and other chemical manufacturing processes. Cyanide (CN-) is evolved during gas manufacture, plating, case hardening and metal cleaning. Nitrogen compounds also originate from explosive factories and other chemical works. There are some cases recorded of severe nitrogen pollution from industrial sources. Haber Bosch process does not only produce ammonia for fertilizer, it also provides ammonia as a raw material to create multiple products. Production of nylon, resins, plastics and melamine require ammonia [163]. Approximately 23 Tg N produced from Haber Bosch process was used for chemical production [164]. Unfortunately, little is known about the fate of this nitrogen in the environment.
Severe ammonia pollution in the Tolka River in Ireland is due to poor discharge qualities of Clonee, Co [165]. The pollution caused chemical burn to the vegetation, absence of aquatic flora and fauna, shortness of breath, skin and eye irritation and live fish and invertebrates were not observed in that area. Thousands of dead fishes were also observed. Nitrogen inputs to rivers, lakes and ocean originate either from point or non-point sources (Table 23) [166]. The point sources of nitrogen pollution include effluent pipes from municipal sewage treatment plant and factories. The non-point sources include the runoff from urban runoff from areas having a population of less than 100,000. These point sources are continuous and can be easily identified, monitored and controled. The non-point sources arise from suite of activities across large areas and are difficult to control [166,167].
| Point Sources | Nonpoint Sources |
| Wastewater effluent, both municipal and industrial | Runoff from agriculture (including return flow from irrigated agriculture) |
| Runoff and leachate from waste disposal site | Runoff from pasture and range |
| Runoff and infiltration from animal feed lots | Urban runoff from unsewered areas and seweredaread with a population of less than 100,000 |
| Runoff from mines, oil fields and unsewered industrial sites | Septic leachate and runoff from failed specifications |
| Strom sewer outfalls from cities with a population of greater than 100,000 | Runoff from construction sites smaller than two hectares and abandoned mines |
| Runoff from construction sites larger than two hectares | Atmospheric deposition over a water surface |
| Overflows of combined storm and sanitary sewers | Activities on land that generate contaminants, such as logging, wetland conservation, construction and development of land or waterways |
Table 23: Sources of point and nonpoint pollution [166].
Rouse et al. [168] reported that industrial effluents and wastewater treatment plant discharges from urban areas are a substantial source of nitrogen to aquatic ecosystems. Initially, these discharges contribute only a small percentage of total nitrogen released to the environment but long term discharges into the water systems cause detrimental effect on stream ecosystems.
Another source of nitrogen contaminations in the urban areas is precipitation. Rain, snow and fog contain various amounts of nitrogen. Motor vehicles and industrial exhausts contribute nitrogen oxides (NO and NO2) to the atmosphere which are deposited in the water systems through precipitation. Precipitation causes problem in the watersheds which do not have an extensive ground cover of natural vegetation such as in urban areas [169].
Lake eutrophication
Eutrophication was considered a natural biological aging process of aquatic ecosystems where nutrients increase produce more plants leading to the formation of pond and finally into a marsh [170]. However, the term eutrophication is currently used to describe a rapid nutrient enrichment process that take place in water bodies as a result of human activities that add up the nutrients. Present knowledge indicates that the fertilizing elements most responsible for Lake Eutrophication are nitrogen and phosphorus. Iron, silica and certain trace element are also important [171].
Although the increase in yield of a crop after fertilization is desirable in terrestrial situations, the effects of eutrophication of waters are undesirable. Generally, the aesthetic value of a lake is lowered through excessive growth of aquatic weeds and algae and production of floating algal scums which are a nuisance to those who used the water for recreational purposes. Other effects include undesirable odors and tastes, depletion of dissolved oxygen, destruction of aquatic life and impairment of water treatment operations such as clogging of filters by algae [172,173].
The stage of lake eutrophication is not controlled solely by the quantities of nutrients present or entering the receiving body of water, but also the interrelationship of climatic, physical, biological and chemical factors which affect lake metabolism are very important and significant and must be considered. However, nitrogen is the most important nutrient that limits primary production of photosynthetic organisms in temperate and coastal marine waters [171]. At the estuarine area, nitrogen often becomes the first nutrient to limit primary production. Excess inorganic nitrogen that flows to the estuarine will support a larger bloom in that area [171-177].
Different forms of nitrogen compound (organic or inorganic) reach the streams and subsequently the lakes through: (a) surface runoff, (b) groundwater interflow and (c) domestic and industrial wastes disposed of into the river. In the last four decades, nitrogen flux entering Mississippi River in USA had increased four folds and more than ten folds into the North Sea [65,150,178].
Besides agricultural activities, aquaculture industry (growing resources) also contributes nutrients to streams. Fish farms in developing countries are often located in a shallow bay area, allowing nutrients to accumulate and stimulate algal growth [179]. Romdhane et al. [180] described aquaculture activities in lagoons as a hazardous business as lagoons may function as a trap for toxins or other exudates from algal.
Other source of nutrients includes nitrous oxides in wet and dry deposition. About 50-100% primary production of harmful algal blooms in Yellow Sea, China is generated by typical rain event containing nitrogen, phosphorus and silica [181].
The level of nitrogen which will produce algae blooming is quite varied and depends on many factors, the most important of which are the available phosphorus and organic carbon. Nutrient ratios play an important role in influencing the algal growth. Hodgkiss et al. [182] reported algal bloom in Tolo Harbour in Hong Kong increases whenever the ratio N: P decreases. The authors recorded that the ration of N: P between 1982 and 1989 in Tolo Harbour and found it to decreases from 20:1 to 11:1 as the number of dinoflagellate red tides increased. Hodgkiss [183] demonstrated that whenever N: P ratio fell below approximately 10:1 in Tolo Harbour, the dinoflagellate cell number increased. Algal bloom of dinoflagellate red tides result in prominent colouration of the water bodies. Microflagella dinoflagella blooms when nitrogen concentration is low while some other species such as marine diatoms exploits nitrate-rich conditions through physiological adaptations [184-197].
Smith et al. [188] reported that in Northern Ireland, soil P reserves have accumulated at a rate of 1000 kg P km-2 yr-1 over the past 50 years and these increases have resulted in increases in the losses of inorganic P runoff at a rate of 2 mg m-3 yr-1. The average of characteristics of water bodies with various levels of nitrogen and phosphorous loading is shown in Table 24 [160]. McGarrigle [189] reported that annual dissolved inorganic P concentration should be lessern tha 47 mg m-3 to prevent the growth of algae and to preserve water quality suitable for salmonoid fish, in Irish rivers. Miltner et al. [190] reported that the total inorganic nitrogen (TIN) and dissolved inorganic phosphorus (SRP) concentrations exceeded 610 and 60 mg m-3, respectively causing deleterious effects to marine organisms in Ohio streams, USA.
| Water bodies | Trophic state | Total nitrogen (mg m-3) | Total phosphorus (mg m-3) | Chl a (mg m-3) |
| Lakes | Oligotrophic | <350 | <10 | <3.5 |
| Mesotrophic | 35-650 | 10-30 | 3.5-9 | |
| Eutrophic | 650-1200 | 30-100 | 9-25 | |
| Hypertropic | >1200 | >100 | >25 | |
| Streams | Oligotrophic | <700 | <25 | <10 |
| Mesotrophic | 70-1500 | 25-75 | 10-30 | |
| Eutrophic | >1500 | >75 | >30 | |
| Marine | Oligotrophic | <260 | <10 | <1 |
| Mesotrophic | 260-350 | 10-30 | 1-3 | |
| Eutrophic | 350-400 | 30-40 | 3-5 | |
| Hypertropic | >400 | >40 | >5 |
Table 24: Average characteristics of lakes, streams and marine waters [188].
Xiangcan et al. [172] studied the eutrophication in various freshwater lakes in China and reported that a total nitrogen concentration of 5.45 mg/L in Lake Nanhu which is higher than the permitted level of 0.6 mg/L. The author also reported that the total phosphorous in Lake Dianchi was 0.529 mg/L which is higher than the permitted level of 0.02 mg/L.
Anderson et al. [191] reported that nitrogen and phosphorus are two major limiting factors in water bodies. In freshwaters, phosphorous are least available in large quantities for the photosynthetic organisms and can limit or co-limit the growth of algae in estuarine and marine environments that are sustaining high nitrogen inputs. In some tropical and highly eutrophic temperate lakes, nitrogen may be a more important limiting factor than phosphorus. Duan et al. [192] reported that the nutrient sources for eutrophication are mainly sewerage, livestock drainage, soil nutrients and loss of fertilizers in drained agricultural lands, which could be associated with human population and economic development.
Mackenthum [193] reported that a concentration of 0.30 mg/L inorganic nitrogen is considered critical for stimulation of algal growth in the presence of adequate phosphorus in Lake Michigan, where total inorganic nitrogen averaged 1.56 mg/L and reached as high as 3.14 mg/L in Indiana Harbor. The source of pollution in this example was from the discharged domestic waste. However, excessive growth of plants and algae in polluted water can be avoided if the concentration of nitrate nitrogen is kept below about 0.3 mg/L and the concentration of total nitrogen is not allowed to rise much more above 0.6 mg/L. Certain species of algal are classified as harmful algal blooms because toxic compounds are released from this type of algal. Anderson [194] suggested that there is a relation between the toxicity of the harmful algal blooms with nutrients content in the water. Toxicity can increase or decrease significantly depending on the limiting nutrients of the species. For example, saxitoxin production by A. tamarense can be 5-10 folds higher in phosphorus limited environment in comparison to nitrogen limited environment [194,195].
The algal bloom may affect fish, by lowering the dissolved oxygen (DO) in water during the night. This algae oxygen demand can deplete the DO sufficiently and lead to fish kills. Fish may also find it hard to feed if the algae color the water and obscure their vision. Algal blooming at the surface of water will block the sunlight from reaching the bottom of the water bodies. Lack of sunlight can disturb the photosynthesis process of plankton and may lead to another ecological problems such as loss of biodiversity [6,196].
Carpenter et al. [166] reported that the adverse effects of eutrophication are: (a) increased phytoplankton biomass, (b) shifts in phytoplankton to bloom forming species which are toxic or inedible, (c) increases in blooms of gelatinous zooplankton in marine environment, (d) increases in biomass of benthic and ephiphytic alage, (e) changes in macrophyte species composition biomass, (f) death of coral reefs, (g) decreased transparency of water, (h) depleted oxygen levels, (i) increased incidence of fish kills and (j) reduced harvest of fish and shellfish.
Human and animals health
Webb et al. [197] reported that animal wastes are rich in organics and high in biochemical oxygen-demand (BOD). The treated human sewage contains 20–60 mg BOD/L, raw sewage contains 300–400 mg BOD/L, and swine waste slurry contains 20,000–30,000 mg BOD/L. Skinner et al. [198] reported that agricultural wastes such as cattle slurry contain 50 times greater BOD (10,000-20,000 mg/l) than domestic sewage. The ranges of BOD concentrations for various wastes in shown in Table 25 [111].
| Source | BOD (mg/L) |
| Silage effluents | 30,000-80,000 |
| Pig slurry | 20,000-30,000 |
| Cattle slurry | 10,000-20,000 |
| Liquid effluents draining from slurry stores | 1000-12000 |
| Dilute dairy parlour and yard washing (dirty water) | 1000-5000 |
| Milk | 140,000 |
| Untreated domestic sewage | 300-00 |
| Treated domestic sewage | 20-60 |
| Clean river water | <5 |
Table 25: Ranges of BOD concentrations for various wastes [111].
Burkholder et al. [131] and Mellon et al. [199] reported that animal wastes carry parasites, viruses, and bacteria as high as 1 billion/g. Swine wastes contain >100 microbial pathogens that can cause human illness and disease. About one-third of the antibiotics used in the United States each year is routinely added to animal feed to increase growth. This practice is promoting increased antibiotic resistance among the microbial populations present and, potentially, increased resistance of naturally occurring pathogens in surface waters that receive a portion of the wastes. The pathogens in the animal manure are capable of causing various diseases to humans. Some of the select pathogens found in animal manure are shown in Table 26 [125].
| Pathogen | Disease | Symptoms |
| Bacillus anthracis | Anthrax | Skin sores, headache, fever, chills, nausea, vomiting |
| Escherichia coli | Colibacilosis, Coliform mastitis-metris | Diarrhea, abdominal gas |
| Leptospirapomona | Leptospirosis | Abdominal pain, muscle pain, vomiting, fever |
| Listeria monocytogenes | Listerosis | Fever, fatigue, nausea, vomiting, diarrhea |
| Salmonella species | Salmonellosis | Abdominal pain, diarrhea, nausea, chills, fever, headache |
| Clostirdumtetani | Tetanus | Violent muscle spasms, lockjaw, difficulty breathing |
| Histoplasmacapsulatum | Histoplasmosis | Fever, chills, muscle ache, cough rash, joint pain and stiffness |
| Microsporumand Trichophyton | Ringworm | Itching, rash |
| Giardia lamblia | Giardiasis | Diarrhea, abdominal pain, abdominal gas, nausea, vomiting, fever |
| Cryptosporidium species | Cryptosporidosis | Diarrhea, dehydration, weakness, abdominal cramping |
Table 26: Pathogens found in animal manure [115].
Burkholder et al. [131] reported that waterborne contaminants affect human health from both recreational use of affected surface water and from ingestion of drinking water derived from either contaminated surface water or groundwater. Generally, the very young, elderly, pregnant women and immune compromised individuals are at great risk of infection. Accidental ingestion of contaminated water that may result in diarrhoea or other gastrointestinal tract distress from waterborne pathogens and dermal contact during swimming that may cause skin, eye, or ear infections. Drinking water exposures to pathogens could occur in vulnerable private wells; under normal circumstances community water utilities disinfect water sufficiently before distribution to customers.
WHO [200] and Carmichael et al. [201] reported that cyanobacteria (blue green algae) released from animal manure into the surface can produce toxins such as microcystins that are neurotoxins and hepatotoxins. Acute and chronic health impacts are bound to occur on exposure to these toxins.
High concentrations of nitrites in water consumed by infant causes methemoglobinemia. Heldin [202] reports that methemoglobinemia was found in young bottle-fed infants in parts of Neapawa and Portage le Prairie in Manitoba, Canada, and Health Service traced it to be caused by high nitrate content of water in local wells. Also, at about the same time, nitrate poisoning of cattle was reported by local veterinarian. High nitrate in forage can be contributing factor in livestock production. The drinking water standards (1962) of the U.S. Public Health Service list 10 ppm nitrate nitrogen (45 ppm NO3) as the level that should not be exceeded for infants.
Cornell et al. [203] reported that applying synthetic nitrogenous fertilizer onto land has pronounced effect on the local and global environment. Ammonia (NH3), nitrous oxide (N2O) and nitric oxide (NO) are the main nitrogen gas species emitted to the atmosphere from agricultural activities. Nitrous oxide (N2O) and nitric oxide (NO) are very minor contributors, but ammonia (NH3) contributes significantly to the atmospheric nitrogen deposition. Ammonia and nitrogen oxides present in the atmosphere are removed by rainfall and dry deposition [204].
Camargo et al. [205] reported that some algal species can cause toxicity to aquatic and terrestrial animals by synthesis of certain toxin such as anatoxins, microcystins, nodularins, brevetoxins, hemolysins and saxitoxins (Table 27) [205]. These toxins remain can inside the algal cells (intracellular toxins) or can be released into the water (extracellular toxins) during active algal growth or when cells lyse. These toxins are consumed by animals by direct exposure to water, drinking of water or injestion of algal cells from water.
| Toxins | Chemical Structure | Site and mode of action | Characteristic species |
| Anatoxin-a | Secondary amine alkaloid | Attacks nervous system by mimicking the action of acetylcholine and over stimulating muscle cells | Anabaena circinalis, Anabaena flos-aquae, Anabaena planctonica, Aphanizomenonflos-aquae |
| Anatoxin-a (s) | Organophosphate | Attacks nervous system by inhibiting acetyl-cholinesterase, and over stimulating muscle cells | Anabaena flos-aquae, Anabaena lemmermannii |
| Brevetoxins | Polycyclic ethers | Attacksnervous system by binding to sodium channels and disrupting nerve conduction | Kareniabrevis |
| Domoic acid | Tricarboxylic amino acid | Attacks nervous system by binding to kainate glutamate receptors, and causing depolarization of the neurons, with a subsequent increase in intracellular Ca2+, neuronal swelling, and cell death | Pseudo-nitzschiaaustralis, Pseudo-nitzschiadelicatissima, Pseudo-nitzschiamultiseries, Pseudo-nitzschiaseriata |
| Hemolysins | Fatty acids | Target cells by altering membrane functions and causing cell lysis | Alexandriummonilatum, Gymnodiniumaureolum, Kareniamikimotoi |
| Microcystins | Cyclic heptapeptides | Affects liver, hepatopancreas by shrinking the cytoskeleton, distorting cells and causing hepatic haemorrhages, and by inhibiting protein phosphatases, and causing tumor promotion | Anabaena flos-aquae, Microcystisaeruginosa, Microcystisviridis, Planktothrixagardhii, Planktothrixrubescens |
| Nodularins | Cyclic pentapeptides | Affects liver, hepatopancreas by shrinking the cytoskeleton, distorting cells and causing hepatic haemorrhages, and by inhibiting protein phosphatases, and causing tumor promotion | Nodulariaspumigena |
| Saxitoxins | Carbamate alkaloids | Attacks nervous system by blocking sodium channels and disrupting nerve conduction | Anabaena circinalis, Aphanizomenonflos-aquae, Alexandriumcatenella, Alexandriumtamarense, Gymnodiniumcatenatum |
Table 27: Impact of toxins from algae on aquatic and terrestrial animals [205].
Animal waste treatment
In order to reduce the amount of nitrogen compound in animal waste, manure is to be collected and treated in such a way as to make aerobic conditions prevail. Aerobic treatment will encourage the conversion of ammonium nitrogen into nitrate nitrogen through the process of nitrification. When the aerobic process is followed by anaerobic conditions, denitrification will be encouraged resulting in stripping out the elemental nitrogen to the atmosphere leaving the treated manure with a lower content of nitrate [141].
Another method is the ammonia removal from units holding animal wastewater. The ammonia release to the atmosphere is a function of the agitation. Aeration towers can be used to degasify animal waste. Because holding tanks are prevalent at animal production facilities, aeration systems can be used for ammonia release, where the amount of ammonia stripped from a solution is a function of the air flow rate, temperature, pH of the liquid, time and characteristics of waste water [206].
The ultimate utilization of animal manure will be land application. If nitrogen reduction is a goal in waste disposal, a rather wet schedule should be followed [207]. The applied waste water should contain some organic load so that a carbon substrate would be available for denitrifying bacteria. Once application of wastewater has begun on an area, it should be continued for as long as possible to ensure conversion of ammonia nitrogen to nitrate so that maximum reduction of nitrogen can be achieved by denitrification.
Proper storage and application of slurry and solid manure can help reduce emissions [208]. Rapid incorporation of slurry and solid manure into the soil is the simple way to reduce ammonia emission. Band spreading machineries such as trailing house and trailing shoe and sub-surface applicators reduces ammonia emissions by 30-40% compared with broadcast spreading. Shallow injection and deep injection can reduce ammonia emissions by 30-40% and 90%, respectively. The trailing house and subsurface applicators are shown in Figure 7 [207,208]. Some other additional methods of ammonia abatement are shown in Table 28 [208].
| Method | Mode of action | Disadvantages |
| Reduce crude protein in livestock diet | Reduces amount of N excreted and hence potential for ammonia emission | More research needed |
| Use extra straw for bedding cattle | Locks up ammonia | Research ongoing |
| Modify design for livestock housing | Reduces time and area of slurry exposed to air | Mainly for pigs. Difficult to implement for existing houses |
| Equip livestock houses with air scrubbers or filters | Removes ammonia from air ventilated from houses | Only for mechanical ventilated houses and it is very expensive |
| Cover surface of store with straw plastic sheet, clay granules, oil etc. | Physical barrier to ammonia emission | Can be unreliable. Granules or straw may block pumps etc. |
| Additives (eg. Zeolites) | Physically locks up ammonia or form stable chemical compound | Large quantities needed and not reliable |
| Dilute slurry with water | Speeds up infiltration into the soil | Effectiveness varies with soil conditions. Increases volume of slurry to be managed |
| Add acid to slurry | Lowers pH and so ammonia stays in solution | Hazardous and can release nitrous oxide |
| Remove proportion of solids from slurry with a mechanical separating machine | More free flowing liquid infiltrates into the soil more rapidly | Specialist machinery required and there are ammonia emissions form the remaining solid material |
| Irrigate with water after spreading slurry | Washes slurry into the soil | Needs large amounts of water and may cause run off to surface waters |
| Choose optimum time | Cool, humid weather discourages ammonia releases rain washes slurry into the soil | Difficult to quantify effectiveness. May cause odour problems |
| Match fertilizer type and use of crop need, soil and environmental conditions | Urea is one of fertilizers with highest ammonia emissions | Requires more time on fertilizer planning |
Table 28: Additional methods for ammonia abatement [208].
Slurry storage plays an important role in ammonia emission. Specifically made round covers fitted to above ground tanks and slurry lagoons can reduce ammonia emissions by up to 80%. In addition potentially cheaper covers such as LECA (light expanded clay aggregate) or UV stabilized plastic sheets provide significant reduction in ammonia emissions stored from pig slurry. The different emission control options for cattle and pig slurry storage is shown in Table 29 [208-210].
| Methods | Emission reduction efficiency (%) | Applicability |
| Store with no cover or crust | 0 | |
| Tight lid, roof or tent structure | 80 | Concrete or steel tanks and silos. May not be suitable on existing storage tanks |
| Plastic sheeting (floating cover) | 60 | Small earth-banked lagoons |
| Allowing formation of natural crust by reducing mixing and manure input below the surface | 40 | Only for slurries with higher content of fibrous material. Not suitable on farms where it is necessary to mix and disturb the crust in order to spread slurry frequently. Crust may not form on pig manure in cool climates |
| Replacement of lagoon, etc. with covered tank or tall open tanks (depth >3m) | 30-60 | Only new build and subject to any planning restrictions concerning taller structures |
| Storage bag | 100 | Available bag sites may limit use on larger livestock farms |
| Floating LECA balls (Hexa covers) | 60 | Not suitable for crusting manures |
| Plastic sheeting (floating cover) | 60 | Large earth blended lagoons and concrete or steel tanks. Management and other factors may limit use of this technique |
| Low technology floating covers (eg.: chopped straw, peat, bark) | 40 | Concrete or steel tanks and silos. Probably not practicable on large earth banked lagoons. Not suitable if materials likely cause slurry management problems |
Table 29: Storage methods for the reduction of ammonia emission [210].
Domestic waste treatment
The use of nitrification-denitrification as a method for eliminating nitrogen contamination by waste water was examined by Mulbarger [211]. The process alternatives for the nitrification-denitrification process included: (a) substituting trickling filter for the high rate and/or nitrifying activates sludge systems, (b) adding inorganic or organic coagulants to primary clarifier to maximise solids removal and eliminate the high rate activated sludge systems and (c) utilizing anaerobic column or beds for denitrification. The whole process was carried out in a single, dual and three sludge systems. The author reported that an influent with 25.2 mg/L total nitrogen after treatment using the system produced an effluent with 5.4 mg/L total nitrogen (1.4 mg/L nitrate).
Strous et al. [212] studied the ammonium removal from concentrated waste streams with anaerobic ammonium oxidation (anammox) process. In this process, the ammonium was converted to dinitrogen gas with nitrite as the electron acceptor. The process is autotrophic and there was no need for COD addition to support denitrification. When the anammox process was preceded by a nitrification step, only a part of ammonium was nitrified and the anammox process combines the ammonium and nitrite to form dinitrogen gas. This reduced the oxygen demand and the biomass yield was very low. This approach reduces the total operation costs [213,214]. Strous et al. [212] studied the effects of sludge digestion effluent on waste streams in a fluidised bed reactor using anammox process. The whole process was carried out for 150 days at a pH of 8 and a temperature of 36°C. The results indicated that during 150 days of analysis, 82% of ammonium and 99% nitrite were removed in fluidized bed reactor. Dong et al. [215] studied the anaerobic digestion of poultry manure using anammox and denitrification processes. The results obtained from the study indicated that the anammox microorganisms developed at a very low rate and could not compete with the denitrifying bacteria for nitrite production.
Liao et al. [216] studied the removal of nitrogen from swine manure wastewaters by air (ammonia) stripping and aeration methods. The air stripping process took place in a stripping tower in which the wastewater was pumped in from the top which passed through the packing material and the air was blown from the opposite direction to release volatile ammonia in the air. The direct aeration involved pumped air directly into the reactor containing swine manure wastewater to release ammonia to the air. The results indicated that the air stripping method achieved 90% ammonia removal at a pH of 11.5 after 1 h of treatment and the aeration method achieved 90% removal at a pH of 11.5 after 150 h of treatment.
Crop production practice
The use of nitrogen fertilizers is recommended to be at the optimum level required by the crop and soil type in order to eliminate addition of any excess. Land use practice and soil conservation methods that reduce the amount of erosion will consequently reduce the amount of organic nitrogen carried to the surface waters.
Flynn [217] recommended management strategies to improve the nitrogen use efficiency of crops, thereby reducing fertilizer requirements and associated greenhouse gas emissions. The author suggested that both environmental factors such as soil conditions and climate and management factors such as tillage play an important role in the amount of fertilizer to be applied. Appropriate nitrogen application rates are required to limit the buildup of nitrates in soil. Organic nitrogen sources such as animal manure, crop residues and nitrogen fixing crops can be used instead of synthetic fertilizer.
Tilman et al. [218] reported that nutrient use efficiency must be improved by better matching temporal and spatial nutrient supply with plant demand. Practices such as applying fertilizers during periods of greatest crop demand at or near the plant roots and smaller and frequent application of nutrients have the potential to reduce the nitrogen emissions and improving the yield and quality of the crops. This type of agriculture is called precision agriculture and it is currently carried out in large scale intensive farming which could be implemented on small level farming.
Multiple cropping systems such as using crop rotations or intercropping (two or more crops grown simultaneously) can help to improve pest control and increase nutrient and water use efficiency. Crops such as clover and other legumes can be in rotations to reduce fertilizer requirements by adding biologically fixed nitrogen into soils. Addition of post-harvest plants remains into the soil can increase the levels of soil organic matter and help in storing soil carbon. Agroforestry, in which growing trees can improve nutrient availability and efficiency and can reduce erosion in the soil [217,218].
Changing from fall nitrogen application to a spring application will reduce nitrous oxide emissions [219]. In Alberta cropping systems, between 30-50% nitrogen emissions have been reduced by changing fertilizer application methods. Increasing the acreage of minimum tillage also reduced the carbon di-oxide emissions from the soil and increased the carbon sequestration in soil. Naylor [220] reported that technologies such as drip irrigation can improve water use efficiency and decrease salinization in the soil. The sustainable growing of crops will require increased efficiency of nitrogen and water use along with ecologically based best management practices and effective use of pesticides.
The natural cycle of nitrogen involves several biological and non-biological process including: mineralization, nitrification, denitrification, nitrogen fixation, microbial and plant uptake of nitrogen, ammonia volatilization, leaching of nitrite and nitrate and ammonia fixation. Nitrogen exists naturally in the environment and is constantly being converted from organic to an inorganic form and vice versa. Production of commercial fertilizer adds up to the natural source of nitrogen. The main source of nitrogen include: atmospheric precipitation, geological sources, agricultural land, livestock and poultry operations and urban waste. Agricultural emissions show a strong increase due to the application of fertilizer to agricultural soils, grazing of animals and spreading of animal manure. Emissions from agricultural practices and animal manure wastes are the major source of nitrogen pollution in surface and underground water. Soil erosion and runoff from fertilized land as well as domestic and industrial wastes contribute to the enrichment of lakes and streams with nutrients. Nitrates concentration exceeding certain limits in drinking water is toxic to animals and humans, especially infants. Nuisance of algal bloom and fish kills in lakes and rivers occurs due to eutrophication. Obnoxious colors and smells are developed as a result of organic matter decay and are destroying the natural beauty of the environment. The water born contaminants affect human health from both recreational use of contaminated surface water and from ingestion of contaminated drinking water derived from surface or ground water sources. The methods for abatement of nitrogen pollution must follow multi pathways. First, the source and amount of pollution must be detected and defined. Second, the possible ways to treat animal and domestic wastes should be carefully investigated. Third, better agricultural practices should be developed that include: proper storage and application of slurry and solid manure, rapid incorporation of slurry and solid manure into the soil, use of band spreading machineries such as trailing house and trailing shoe and sub-surface applicators, use of specifically made round covers fitted to above ground tanks and slurry lagoons, applying fertilizers during periods of greatest crop demand at or near the plant roots in smaller amounts with frequent applications, using multiple cropping systems such as using crop rotations or intercropping to increase the efficiency of nitrogen uses and changing current livestock production techniques.
The research was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada.