Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2021)
Background: Hepatitis is an inflammation of the liver, can be self-limiting or progress to fibrosis, cirrhosis, or hepatocellular carcinoma, HB occurs as a result of parenteral contact with infected body fluids, could be vertical from mother to baby or horizontal.
Aim of the study: To assess the effectiveness of HB vaccine alone versus HBIG combined with HB vaccine in the interruption of neonatal HB viral infection.
Methods: A Non-randomized clinical trial was conducted, 228 subjects distributed into two groups, the control group: newborns of mothers with inactive HBV infection were given HBIG and HB vaccine and, the intervention group: Newborns of mothers with inactive HBV infection were given HB vaccine alone.
Results: showed that the two immunization regimens were effective in preventing HB vertical infection, GMT of the infants who vaccinated with HB vaccine alone (207.64 IU/L) higher than the infants who vaccinated with HB vaccine combined with HBIG (180.87 IU/L), the overall non-protective rate was 6.6% (15/228), (7.89%) among the control group compared to (5.26%) among the intervention group, RR 2.63, HBV incidence rate was zero.
Conclusion: HB vaccine alone completely prevents HBV vertical infection and it`s not inferior to HB vaccine combined with HBIG.
Prevention; HBV infection; HBIG, HB vaccine; GMT
Hepatitis is an inflammation of the liver, the condition can be selflimiting or can progress to fibrosis, cirrhosis or liver cancer. Hepatitis viruses are the most common cause of hepatitis in the world but other infections, toxic substances (eg. alcohol, certain drugs), and autoimmune diseases can also cause hepatitis [1]. There are 5 main hepatitis viruses, referred to as types A, B, C, D and E [2,3]. Hepatitis B (HB) C and D usually occur as a result of parenteral contact with infected body fluids. Common modes of transmission for these viruses include receipt of contaminated blood or blood products, invasive medical procedures using contaminated equipment and for HB transmission from mother to baby at birth, from family member to child, and also by sexual contact [2-4]. HB has been characterized as a “silent disease”; worldwide, chronic HB infection is the sixth leading cause of death [5-7].
Study setting
The study was conducted in two localities in Gaza Strip (GS), the first was the Epidemiology departments, part of the preventive medicine directorate in the Primary Health Care (PHC) directorate in the Palestinian Ministry of Health (PMOH). There are five departments in GS. The second setting was the delivery rooms in the governmental hospitals in GS. The hospitals included in the study were Al-Shifa hospital, The European Gaza hospital and Nasser hospitals.
Study design
Non-randomized clinical trial (non-inferiority study). The study was conducted along two groups, control group: Newborns of mothers with inactive HBV infection were given HBIG and HB vaccine and intervention group: Newborns of mothers with inactive HBV infection were given HB vaccine alone.
Target population
HBV infected mothers and their newborns were included in the study.
Sample size and sampling design
Sample size: Aimed for 90% power at 5% significance level, and assumed an event rate of 80% in the control arm (proportion of immune infants who were born to HBV negative mothers and who have received HBV vaccine alone), 114 subjects were included per arm for corrected chi-square and Fisher's exact tests to detect a difference of 15% in proportion of immune infants between the two arms. This sample size has had enough statistical power to detect a difference of 15% in the secondary end point as well. The study sample was calculated using Stats-Direct statistical software, Version 2.8.0, 2013 [8].
Type of sample and method of selection
Consecutive pregnant females attended the delivery ward of the three hospitals and the patients who attended the epidemiology departments in GS governorates, all were screened for HBV infection. Blood samples were withdrawn from the mothers and were tested for HBsAg, HBeAg and Liver Function Test (LFT). These samples were withdrawn at perinatal period. Blood samples were withdrawn from the infants after 9 months of age and all tested for, HBsAg, Anti-HBs titer.
Data collection methods and tools
This step included Blood sampling for serological testing for HBsAg, HBeAg and LFT from the mother’s pre-delivery. Blood samples were withdrawn from the mothers and tested for HBsAg, HBeAg and LFT. These samples were withdrawn pre-delivery and according to results the researcher had decided who subjects were included in the study. Only the newborns to mothers positive for HBsAg and negative for HBeAg were included in the study. It included also immunization of the newborns with HB vaccine with/without combination of HBIG hastily post-delivery. Immunization with HB vaccine only to 114 newborns their mothers positive for HBsAg and negative for HBeAg. This immunization regimen was adopted at the epidemiology departments. Immunization with HB vaccine combined with HBIG to 114 newborns their mothers positive for HBsAg and negative for HBeAg. This immunization regimen was adopted at the delivery rooms in the hospitals. When infant is 9 months, Blood sampling for serological testing for HBsAg and Anti-HBs titer was done. All of the infants tested for HBsAg in order to identify the rate of HBsAg infection if any and HBsAb with the level of titration in order to identify the efficacy and immunogenicity of the immunization regimen.
Data entry and analysis stage
Collected data were reviewed for completeness and accuracy, coded, computed and analyzed using Statistical Package for Social Sciences (SPSS) program version 21. Descriptive statistics were used to describe the study sample using measures of central tendency and measures of dispersion around the mean. The following statistical tests were also used: ANOVA test and T-test, Chi-square (χ2) test, Geometric mean calculated to assess the level of the Anti- HBs titer, Pearson’s correlation test, RR, ARR and incidence rate of non-protected infants were calculated.
All the infants were tested for HBsAg after nine months of age and all of them were negative, indicating that all of them were free from HBV infection. The Anti-HBs titers were normally distributed among the infants with a range of (3-1000 IU/L), it was 402.11 IU/L among the infants were given HB vaccine combined with HBIG in the maternity departments, Geometric Mean Titration (GMT) was 180.87 IU/L which was less than the infants immunized in the epidemiology departments with HB vaccine alone 428.52 IU/L GMT was 207.64 IU/L (Figure 1). With respect to the time of starting the immunization regimen directly post-delivery it was clear that babies received the regimen between 2-24 hours have the highest level of GMT 211.58 IU/L, with very little difference between the other two groups who vaccinated between 1-2 hours or after 24 hours of life with 182.05 IU/L and 184.11 IU/L respectively.
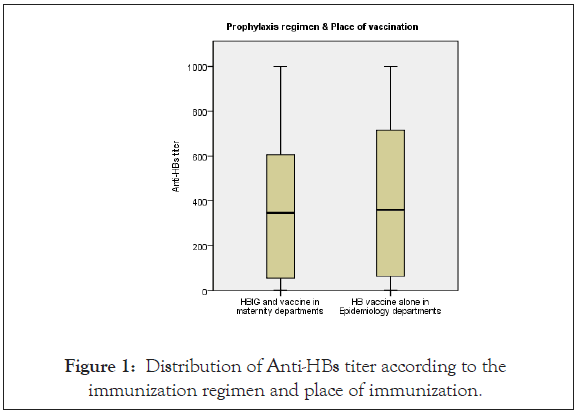
Figure 1: Distribution of Anti-HBs titer according to the immunization regimen and place of immunization.
Both males and females showed completely immune, whereas GMT were among females 154.88 IU/L higher than males 130.1 IU/L. The GMT was the highest among the babies with normal birth weight 197.44 IU/L, then 158.05 IU/L among babies with low-birth-weight 2000-2500 g, while it was the little level 108.67 IU/L among those their birth weights were 4000 g and more.
Among the breast-feeding babies, the GMT was completely immune 197.14 IU/L, while it was non-immune 4 IU/L among non-breast-feeding babies. It was clear that among those starting breast feeding within 3-24 hours post-delivery the GMT was the highest level 345.84 IU/L compared to 190.78 IU/L among those starting breast feeding within the first two hours of life (Table 1).
The Table 1 illustrates that 227/228 (99.5%) of the children breast-fed from their mothers, among them 155/227 (68.3%) were completely immune, 25.6% were relatively immune and 14/227 (6.2%) were non-immune (0-9 IU/L), while one child only did not breast-feed from his/her mother and he/she was completely immune. The difference between the variables was highly significant (0.001) less than 0.05.
| Variable | Anti-HBs titer | 0-9 IU/L | 10-99 IU/L | 100 IU/L and more | Total | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |||
| Breast feeding of the baby | Yes | 14 | 6.2 | 58 | 25.6 | 155 | 68.3 | 227 | 100 | 0.001 |
| No | 1 | 100 | 0 | 0 | 0 | 0 | 1 | 100 | ||
| Total | 15 | 6.6 | 58 | 25.4 | 155 | 68 | 228 | 100 | ||
Table 1: Relationship between the anti-HBs titer and the breast-feeding.
Table 2 shows the relationship between the anti-HBs level of the children and time of starting breast feeding post-delivery, pointing that there were no statistically significant differences between groups as determined by one-way ANOVA (F=1.500, P=0.225).
| ANOVA test | |||||||
|---|---|---|---|---|---|---|---|
| Time in hours of starting breast feeding post-delivery with Anti-HBs titer | Mean | Mean difference | Sig. | F | 95% CI | ||
| Item | Value | Lower | Upper | ||||
| less than 2 hrs | 441.18 | 93.059 | 0.223 | 1.5 | -42.22 | 228.34 | |
| 3 - 24 hours | 348.12 | 99.015 | 0.791 | -266.93 | 464.96 | ||
| 24+ hours | 342.17 | 5.956 | 0.999 | -363.71 | 375.62 | ||
P-value=0.225
Table 2: Difference between anti-HBs titer and time of starting breast feeding post-delivery by using of one-way ANOVA test.
Table 3 illustrates that 114/228 (50%) of the children vaccinated in the governmental hospitals; among them 75/114 (65.8%) were completely immune, 26.3% were relatively immune and 9/114 (7.9%) were non-immune (0-9 IU/L), while among the 114 children vaccinated in the Epidemiology department 80/114 (70.2%) were completely immune, 24.6 were relatively immune and 5.3% were non-immune. This indicates a higher level of responsiveness among the children vaccinated in the Epidemiology departments. The difference between the variables did not reach the statistical significance level (0.660) higher than 0.05.
| Variable | Anti-HBs titer | 0-9 IU/L | 10-99 IU/L | 100 IU/L and more | Total | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |||
| Place of vaccination | Governmental hospitals | 9 | 7.9 | 30 | 26.3 | 75 | 65.8 | 114 | 100 | 0.66 |
| Epidemiology departments | 6 | 5.3 | 28 | 24.6 | 80 | 70.2 | 114 | 100 | ||
| Total | 15 | 6.6 | 58 | 25.4 | 155 | 68 | 228 | 100 | ||
Table 3: Relationship between the anti-HBs titer and the place of vaccination.
Table 4 and 5 shows the relationship between the anti-HBs level of the children and the prophylaxis regimen applied post-delivery. The table points out that the mean of the anti-HBs among the children vaccinated with HB vaccine alone was (428.52) higher than the mean of anti-HBs of the children who were vaccinated with HBIG and HB vaccine (402.11), and this difference between variables did not reach the statistical significance level (0.587).
| Independent samples t-test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Continuous variable | Categorical variable | No. | Mean | SD | Sig. | t value | 95% CI | |||
| Lower | Upper | |||||||||
| Anti-HBs titer | Prophylaxis regimen applied | HBIG and HB vaccine | 114 | 402.11 | 362.666 | 0.587 | -0.544 | -122.05 | 69.226 | |
| HB vaccine alone | 114 | 428.52 | 370.151 | -122.05 | 69.226 | |||||
Table 4: Difference between anti-HBs titer and the prophylaxis regimen applied by using of Independent samples- t test.
| Incidence of non-protection | 0-9 IU/L | RR* | ARR** | CI | ||
|---|---|---|---|---|---|---|
| No. | % | Upper | Lower | |||
| HB vaccine alone | 6 | 5.26 | ||||
| HB vaccine+HBIG | 9 | 7.89 | 1.5 | 33.30% | -3.491 | 2.158 |
| Total | 15 | 6.57 | ||||
*Relative Risk (RR)
**Attributable Risk Reduction (ARR)
Table 5: Incidence rate of non-protection among infants.
The overall incidence of non-protection among both groups was 6.6% (15/228) and it was higher (7.9%) among the infants vaccinated with HB vaccine combined with HBIG compared to the infants vaccinated with HB vaccine alone (5.26%), RR 1.5, ARR 33.3%.
The present study is considered a preliminary non-randomized clinical trial about the prevention of neonatal HBV infection in Palestine.
The study was conducted upon 228 neonates born to inactive HBV infected mothers, 114 to each group, 114 neonates vaccinated with HB vaccine alone in the five epidemiology departments in Gaza governorates and HB vaccine combined with HBIG given to the other 144 neonates in the obstetric departments at Al-Shifa hospital, European Gaza hospital, and Nasser hospital, directly after delivery. Blood testing for HBsAg and Anti- HBs titer was done to all infants after nine months of age.
Those neonates included in the study their mothers were positive for HBsAg and negative for HBeAg, that indicating inactive HBV infection.
Age of the mothers at the time of delivery varies from 21 to 44 years with a mean of 31 years. In this study group of mothers were born after the introduction of HB vaccine to the initial Palestinian immunization program in 1993 indicating that they were not responsive to the vaccination or get the infection even vertically or horizontally [9].
Most of the mothers (98%) completed their pregnancy period while 2% only did not completed the period of pregnancy. 8% of the mothers had spontaneous vaginal delivery, while 16% delivered by Cesarean Section (CS). The Palestinian registers showed that 19.6% were delivered by CS [10,11].
The male:female ratio was 1.1:0.88. Palestinian health indices in 2015 showed that the ratio of 103.3 males to 100 females [12].
The mean weight of the babies were 3117 grams. About all of the babies 99.6% were breast fed by their mothers and most of them 97.3% started breast feeding within the first two hours of life with an average of the first breast-feeding 3.75 hours of life.
All of the babies were vaccinated with HB vaccine even in the epidemiology departments or in the obstetric departments, and half of them (114/228) were given HBIG in the governmental hospitals in GS, and all of them completed the vaccination series. In Palestine, vaccination coverage to all vaccinations according to EPI schedule was 98.9% while in GS it was 90% [11].
All of the study subjects were tested for HBsAg after nine months of age and all of them were negative, indicating that all of the children were free from HBV infection. Most of them 68% had Anti-HBs titer more than 100 IU/L indicating complete immunity, 25.4% had Anti-HBs titer between 10-99 IU/L showing relative immunity, while 6.6% of the children had the Anti-HBs titer between 0-9 IU/L indicating they were non-immune.
The GMT was the highest (197.44 IU/L) and completely immune among all infants with normal birth weight.
Both immunization regimens played a crucial role to protect the infants from being HBV infected and both GMT level reached the completely immune category, and it was higher among those received HB vaccine alone and was vaccinated in the Epidemiology departments 207.64 IU/L, compared to 180.87 IU/L among babies received HB vaccine combined with HBIG and vaccinated in the governmental hospitals in GS, but no statistical difference reached between variables P. value (0.587).
The babies who received the immunization regimen between 2-24 hours have the highest level of GMT 211.58 IU/L, with very little difference between the other two groups who was vaccinated between 1-2 hours or after 24 hours of life and all of them were completely immune; these results indicate that the level of immunity will be more protective when the vaccination to be given directly post-delivery, the relationship between the anti-HBs level of the infants and time of vaccination post-delivery didn’t reach the statistically significant differences between groups as determined by one-way ANOVA (F=0.330, P-value=0.719); This result agreed with Nelson, et, al. that showed timely post-exposure prophylaxis will prevent Mother-To-Child Transmission (MTCT) of HBV infection by 85%-95% [13].
The vaccination of the infants with HB vaccine alone was more protective than the vaccination with HB vaccine combined with HBIG, ARR was 33.3%, CI (-3.491-2.158).
The overall vaccine protection rate was 93.4% (213/228) achieved a protective level of more than 10 IU/L, this result agreed with different studies which revealed that the majority of the infants more than 95% achieved a protective level of Anti-HBs at 7 months of age [14-16].
The study didn`t report any HBV infection case that shows zero HBV incidence rate negative for HBsAg, this indicates the success of the immunization regimens intervened to babies of HBsAg positive and HBeAg negative mothers. In spite of 6.57% (15/228) of infants who didn`t get protective level of Anti-Hbs, the Anti- HBs less than 10 IU/L and more they were negative for HBsAg.
This success could be rationale by the early intervention of immunizing the infants as soon as possible, the efficacy of the immunization regimens, the valuable health education from the clinicians and epidemiologist toward the importance of the early intervention, and the role of immunization toward prevention of HBV infection. It also reveals the familial awareness and the adherence of the hospitals and epidemiology staff with the guideline adopted in the health facilities; this result agreed with the Chinese study in 2011 that reported no one case of HBV infection but 4.3% non-protective rate [14].
The overall incidence of non-protection among both groups was 6.57% and it was higher (7.89%) among the infants vaccinated with HB vaccine combined with HBIG compared to the infants vaccinated with HB vaccine alone (5.26%), RR 1.5, that means the incidence of non-protection in this study was 1.5 times higher among the infants vaccinated with combined regimen than those vaccinated with the vaccine alone.
This proportion of non-protection could be due to breastfeeding which was explicit that 93.3% (14/15) of infants among the group of non-immunes were breast-fed (P-value=0.0010).
No statistically significant differences were found between non- protected infants and other risk factors (familial socio-demographic variables, mother's infection status, mother's age, delivery variables, antenatal care related variables, child's sex, child's weight, type of prophylaxis and place of vaccination).
A number of possibilities account for poor response to HBV vaccination. These include intrauterine infection, vaccine escape mutants, host genetic hypo-responsiveness or non-responsiveness to HBsAg, and immune compromise [17].
The results of the study confirmed that the regimen followed by the epidemiology departments GMT 207.64 IU/L is not inferior to the regimen followed by the hospitals 180.87 IU/L. The infants who vaccinated at the Epidemiology departments with HB vaccine alone was more protective than infants who vaccinated at the hospitals with HB vaccine combined with HBIG, ARR was 33.3%, CI (-3.491-2.158).
The regimen adopted in the epidemiology departments is cost effective compared to the regimen adopted in hospitals. By this study a cost of 11400 $ paid to the 114 infants to offer HBIG doses can be saved to enrich the Palestinian health care system without any negative consequences to the infants immunized with HB vaccine alone, and much more cost had paid and still gets paid by the health system in order to control the infection in-spite of efficacy of the vaccine alone to the same target.
Citation: Ali KAA, Hasab A, Awad N, Abed Y (2021) Non-Randomized Clinical Trial to Interrupt Vertical Hepatitis B Viral Infection. J Antivir Antiretrovir. S20:003.
Received: 05-May-2021 Accepted: 19-May-2021 Published: 26-May-2021 , DOI: 10.35248/1948-5964.21.s20.003
Copyright: © 2021 Ali KAA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.