Journal of Down Syndrome & Chromosome Abnormalities
Open Access
ISSN: 2472-1115
ISSN: 2472-1115
Research - (2020)Volume 6, Issue 2
Background: For many years the numerical abnormalities of chromosomes and their implications in Down, Turner, Patau and other human syndromes have suggested that unknown genes, proteins and enzymes are responsible. In this 22 year-long study, a new approach is described using the electric charge properties of chromosomes to solve issues concerning the mechanism responsible for the development of numerical abnormalities of chromosomes and their implication on the origin, diagnosis, predisposition and prevention of Down syndrome, Turner syndrome, Patau syndrome and other aneuploidies.
Materials and methods: Chromosome material technologies, classical and modern methods, used in this study were provided by the Human Genetics Laboratory, Munroe-Meyer Institute for Genetics and Rehabilitation, University of Nebraska Medical Center, USA.
Results and discussions: The results of this study are radically different from previous studies on numerical abnormalities of chromosomes caused by the non-disjunction and fusion of whole and broken chromosomes. These profound results contribute to a new understanding in the origin, diagnosis, predisposition and prevention of human aneuploidies.
Conclusion: Clinically relevant human syndromes and health conditions have not been fully understood because the electric charge properties of chromosomes, which are responsible for the development of numerical abnormalities of chromosomal, have been doubted, neglected and ignored in genetics and molecular biology where the construction, function and abnormalities of chromosomes are primarily studied. Using chromosome charge, which has been ignored for many years, we present and propose a solution for issues concerning Down syndrome, Turner syndrome, Patau syndrome and other aneuploidies.
Down syndrome, aneuploidy, charge based mechanism, numerical abnormality of chromosomes.
The numerical abnormalities of chromosomes are known as aneuploidy [1]. They are genetic conditions in which the number of chromosomes in the nucleus of a cell deviates from the diploid number due to an extra or missing chromosome. Constitutional aneuploidy, aberrations found at birth, occurs during cell division when the chromosomes of a dividing mother cell do not separate properly between the two daughter cells. For example, in human diploid cells, the typical constitutional number of chromosomes is 46. In an aneuploidy cell, one or more chromosomes are lost (resulting in a modal number of 45 or less) or gained (resulting in 47 or more chromosomes).
Acquired aneuploidy occurs when the number of constitutional chromosomes is changed after the birth of animals and humans. This type of aneuploidy occurs in malignant and other abnormal cells when the normal number of constitutional chromosomes is changed due to chromosome breaks, rearrangements or fusions.
Today, the most common constitutional human aneuploidies containing 47 chromosomes are: trisomy 21 (T21) named Down syndrome (DS) after Dr. Jon Down; trisomy 13 (T13) named Patau syndrome (PS) after Dr. Klaus Patau; trisomy 18 (T18) named Edwards syndrome (ES) after Dr. John; and trisomy 16 syndrome [2]. Among the aneuploidies with 45 chromosomes, the most common and clinically important are: monosomy X syndrome named Turner syndrome (TS) after Dr. Henry Turner [2]; monosomy 8 syndrome; and monosomy 9 syndrome. These are the most commonly distributed and the most extensively studied human syndromes.
Nevertheless, these extensively studied numerical abnormalities are still not fully understood in respect to their mechanism of origin. The main problem is in the understanding of two phenomena known as “non-disjunction” and “fusion” of chromosomes. On the one side, it is known that numerical abnormalities of chromosomes occur when two or more chromosomes are fused permanently or kept together temporarily during non-disjunction [1]. On the other side, the exact mechanism that is responsible for the development of fusion or non-disjunction has not been found. During the last 60 years, researchers working in the fields of biochemistry and molecular biology suggested that genes, proteins and enzymes were the main factor for the development of non- disjunction and fusion of chromosomes. This suggestion became widely known after the sequencing and hybridization of DNA was widely utilized in clinical genetics [3]. Using hybridization and other modern techniques, researchers attempted to identify genes located on human chromosome 21 that would be responsible for the events of non-disjunction and fusion which causes DS [3,4]. Concurrently, research was also being conducted on the mouse chromosome 16, which resembles the human chromosome 21, to further identify DS genes and solve issues concerning the origin of aneuploidy [5,6]. After human genome mapping was conducted on the human chromosome 21 and the chromosome 16 of a mouse, the genes, proteins, enzymes and their mechanisms responsible for the development of non-disjunctions causing DS, TS, PS and aneuploidy were still not identified [6,7]. The exact mechanism responsible for the development of non-disjunction and fusion is still not fully understood. Due to their unknown mechanism of origin DS, TS, PS and other human syndromes are difficult to understand, predict, treat, and prevent.
In this publication, we present some of our novel findings, ideas and proposals concerning the charge based mechanism responsible for the development of numerical abnormalities of chromosomes and their implication in the origin, diagnosis, predisposition and prevention of DS, TS, PS and other human syndromes and aneuploidies.
The chromosome materials and classical and modern methods used in this study were provided by the Human Genetics Laboratory (HGL) at the University of Nebraska Medical Center (UNMC). They are identical with those described in detail in a previous publication [8]. All microphotographs of chromosomes were taken with the microscope “Opton” in magnifications x 1000.
We suspected and studied the electric charge properties of chromosomes as the possible factor for the development of non- disjunction and fusion of chromosomes for several reasons.
First, because both phenomena, the non-disjunction and fusion, were discovered in experimental studies with chromosomes exposed to charge in the form of X-rays. These chromosomes belonged to fruit flies of the genus Drosophila. Their chromosomes (genotype) and morphological characteristics (phenotype) were manipulated by exposure to X-rays in a laboratory organized by Thomas Morgan in 1905. There, genetically manipulated fruit flies and their populations were isolated, propagated and used for different experimental studies conducted by Morgan and his graduate students and staff. From their studies, they discovered the phenomena of non-disjunction [9] and the fusion of chromosomes in the form of ring chromosomes, where chromosomes were abnormally bent and fused at the telomeres [10]. These and other studies made by Morgan and colleagues provided the first discoveries and information for numerical abnormalities of chromosomes.
Secondly, we studied electric properties of chromosomes because charge in the form of X-rays was used for many years as the main factor for causing non-disjunction, fusion and other abnormalities in chromosomes in laboratory research. Many of the most important discoveries in the field of genetics and molecular biology were made from chromosomes that were manipulated by exposing them to X-rays. Some of these discoveries were awarded Nobel Prizes. Using fruit flies exposed to X-rays, Morgan discovered that particles of inheritance known as genes and alleles are located and carried by the chromosomes [11]. For this discovery, he was awarded with the Nobel Prize in 1933. Muller, who joined Morgan’s Drosophila research team, developed a new theory for lethal mutations caused by exposure of animals and humans to X-rays and other sources of ionization and non-ionization radiation [12]. For this discovery, he was awarded with the Nobel Prize in 1946. Using X-rays, Beadle, Tatum and Lederberg made their discovery of the roles in regulating biochemical events within cells through the manipulation of chromosomes in bread mold Neurospora crassa, Escherichia coli and Drosophila melanogaster [13,14]. For this discovery, they received a Nobel Prize in 1958. Using X-ray, Rosalind Franklin obtained images of DNA which paved the way for Watson and Crick to discover the double helix of DNA and receive Nobel Prizes in 1962 [15]. Studying chromosomes of corn (Zea mays) exposed to X-rays, McClintock discovered mobile genetic elements known as “transposons” and “jumping genes” [16]. For this discovery she was awarded with the Nobel Prize in 1983.
Third, we studied electrical properties because charge (in the forms of alpha, beta and gamma radiation) was found to be the main factor for the development of non-disjunction, fusion and other abnormalities of chromosomes of humans, plants and animals. This fact was discovered after the use of nuclear weapons during World War II. The increased incidence of breaks, translocations, fusions and numerical abnormalities of chromosomes were found in plants, animals and humans that were exposed to alpha, beta and gamma radiation after the atomic bombs at Hiroshima and Nagasaki in 1945 [17]. Similar findings were reported in the 1960s when the testing of nuclear weapons was carried out in the Pacific Islands. The local tribes in this area were temporarily moved before and during the testing and after they returned an increased incidence of Trisomy 13 (Patau syndrome) was observed in epidemic proportions [18]. The next discovery was made in chromosomal studies of humans, animals and plants that were exposed to alpha, beta and gamma radiation after the nuclear plant disasters at Chernobyl in the USSR and Fukushima in Japan [19,20].
Our fourth justification for this study was due to the findings that women who were exposed to X-rays for an extensive amount of time, primarily in the abdominal region, developed numerical, constitutional abnormalities such as Trisomy 21 in their subsequent pregnancies [21]. The same researchers also found numerical abnormalities in female mouse ovary cells after radiation with X-rays leading to the phenomenon of “radiation induced non- disjunction” [22].
Lastly, we conducted this study because the electric charge property- related phenomena of chromosomes has been proven as a real scientific phenomenon. We have spent over two decades searching and studying these electric charge properties of chromosomes [8,23]. We suspected and studied satellites, centromeres and telomeres of chromosomes as the main place where events of non- disjunction, fusion and numerical abnormalities of chromosomes developed. We did this deliberately due to the knowledge that satellites, centromeres and telomeres are known to be free of important genes, proteins and enzymes [24,25]. Therefore, events of non-disjunction and fusion cannot be caused by genes, proteins and enzymes. Also, satellites, centromeres and telomeres are known to be rich of tightly coiled highly repetitive heterochromatin containing millions of positively charged histones wrapped with negatively charged DNA [24,25]. Therefore, the charge interaction effects are the primary suspects for the development of non- disjunction and fusion of satellites, centromeres and telomeres.
Using charge of chromosomes we propose solutions concerning the mechanism responsible for the development of non-disjunction and fusion causing numerical abnormalities of chromosomes at the satellites, centromeres and telomeres
Our studies with the electric charge properties of satellites, centromeres and satellites were based on documented information in regards to chromosome charge: electrical charge of satellites has been known for 60 years [26-28]; the charge of the outer and inner pairing domain of centromeres and kinetochores has been known for 30 years [29-35]; and telomeric fusions and ring formations have been known for over 100 years [9,10]. Our studies explain that the normal separation of chromosomes move the two daughter cells when the pooling (moving) forces of the spindle apparatus, located at the microtubules, kinetochore and centromere, are stronger then the attracting forces located at the satellites, centromeres or telomeres holding the chromosomes in association. The non-disjunction event that causes trisomy, monosomy and other aneuploidy occur when the attractive forces located at the satellites, centromeres or telomeres are stronger then the pooling forces located at the microtubule, kinetochore and centromere.
Dependent on the place and conditions where the charge based non-disjunction occurred, trisomies, monosomies and other aneuploidy develop into four main patterns, which are described and illustrated below with original microphotographs and schematic drawings.
Full trisomy caused by the non-disjunction of satellites of acrocentric chromosomes
Acrocentric chromosomes are found in the karyotypes of plants, animals and humans. In a human karyotype, acrocentric chromosomes are numbers 13, 14, 15, 21 and 22. They have different sizes and shapes of satellites located on the top of the short arm (Figure 1). When charged satellites of two or more acrocentric chromosomes are attracted they have the ability to form temporary satellite associations (Figure 2). The number of satellites forming associations are unique between individual patients and our studies found increased numbers of enlarged satellites and satellite association in peripheral blood samples of biological parents of children with T21 (DS) [36,37]. The mechanism of their attraction and non-disjunction is illustrated in
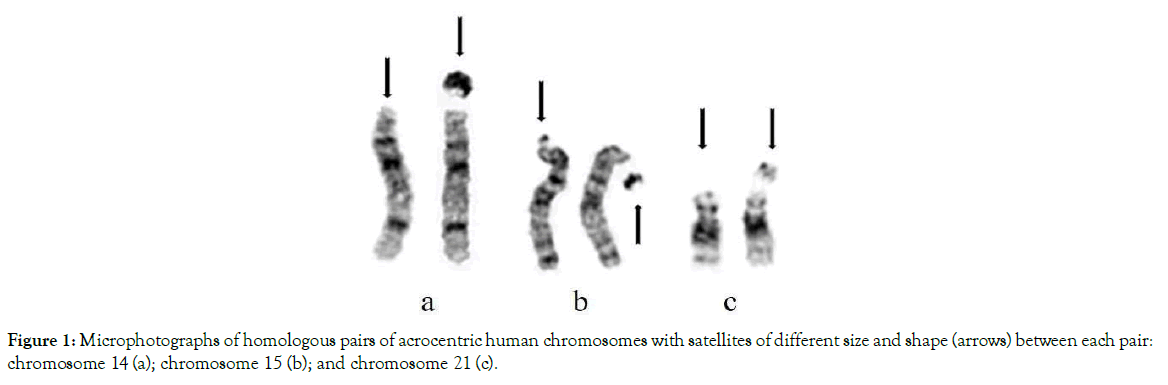
Figure 1. Microphotographs of homologous pairs of acrocentric human chromosomes with satellites of different size and shape (arrows) between each pair: chromosome 14 (a); chromosome 15 (b); and chromosome 21 (c).
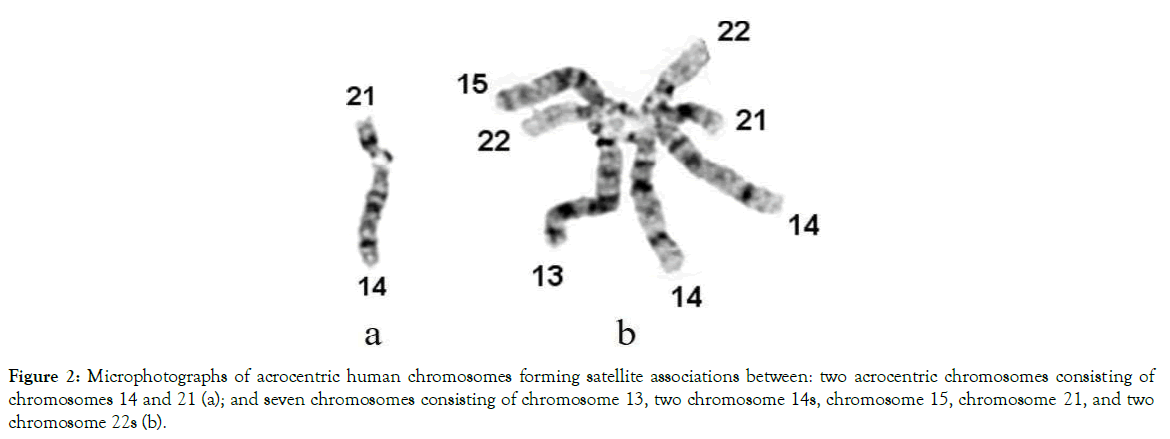
Figure 2. Microphotographs of acrocentric human chromosomes forming satellite associations between: two acrocentric chromosomes consisting of chromosomes 14 and 21 (a); and seven chromosomes consisting of chromosome 13, two chromosome 14s, chromosome 15, chromosome 21, and two chromosome 22s (b).
Full monosomy and trisomy caused by the non-disjunction of centromeres of all chromosomes
The charge based mechanism responsible for the non-disjunction of centromeres is shown in Figure 5. In respect of the autosomal chromosomes, this non-disjunction is associated with the development of constitutional full trisomies of chromosomes 16 (Figure 6a), chromosomes 18, ES (Figure 6b), and chromosomes 8 and 9. From all trisomies and monosomies of constitutional sex chromosomes, the most clinically significant are those of monosomy X known as TS (Figure 6c) and trisomy XXX (Figure 6d), followed by XXY syndrome, known as Klinefelter syndrome, and XYY syndrome.
Double and triple trisomy caused by the non-disjunction of all chromosomes
Double and triple trisomies are found relatively rarely. In our long clinical diagnostic studies with human chromosomes, we have found several cases, two of which are shown in Figure 7. We suggest that the constitutional double or triple trisomies occur from the same charge-based mechanism that is responsible for the development of a single full trisomy of acrocentric, autosomal or sex chromosomes as described above.
Tetrasomy and polysomy caused by the non-disjunction of all chromosomes
Constitutional tetrasomy of chromosome 21 was found in one case (Figure 8). We suggest that the occurrence of tetrasomy and polysomy occur from the same charge-based mechanism that is responsible for the development of a single full trisomy of acrocentric, autosomal or sex chromosomes as described above
Using charge of chromosomes we propose solutions concerning the mechanism responsible for the development of centric fusion translocations, telomeric associations and ring formations causing numerical abnormalities of chromosomes at the centromeres and telomeres
Depending on the place and conditions where the charge based centric fusion translocations, telomeric associations and ring formation occurred, trisomies, monosomies and other aneuploidy developed in seven main patterns. They are described and illustrated below with original microphotographs and schematic drawings.
Trisomy caused by centric fusion (Robertsonian) translocation of acrocentric chromosomes
The first centric fusion of chromosomes was discovered by Robertson [38]. He described and illustrated a V-shaped chromosome which consisted of a centric fusion translocation involving the long arms of acrocentric chromosomes belonging to the grasshopper (Omocestus viridulus). Today, centric fusion translocations of acrocentric chromosomes are found in chromosomes of plants, animals and humans. Named after the researcher who discover them they are known as “Robertsonian translocations” (Figures 3-8).
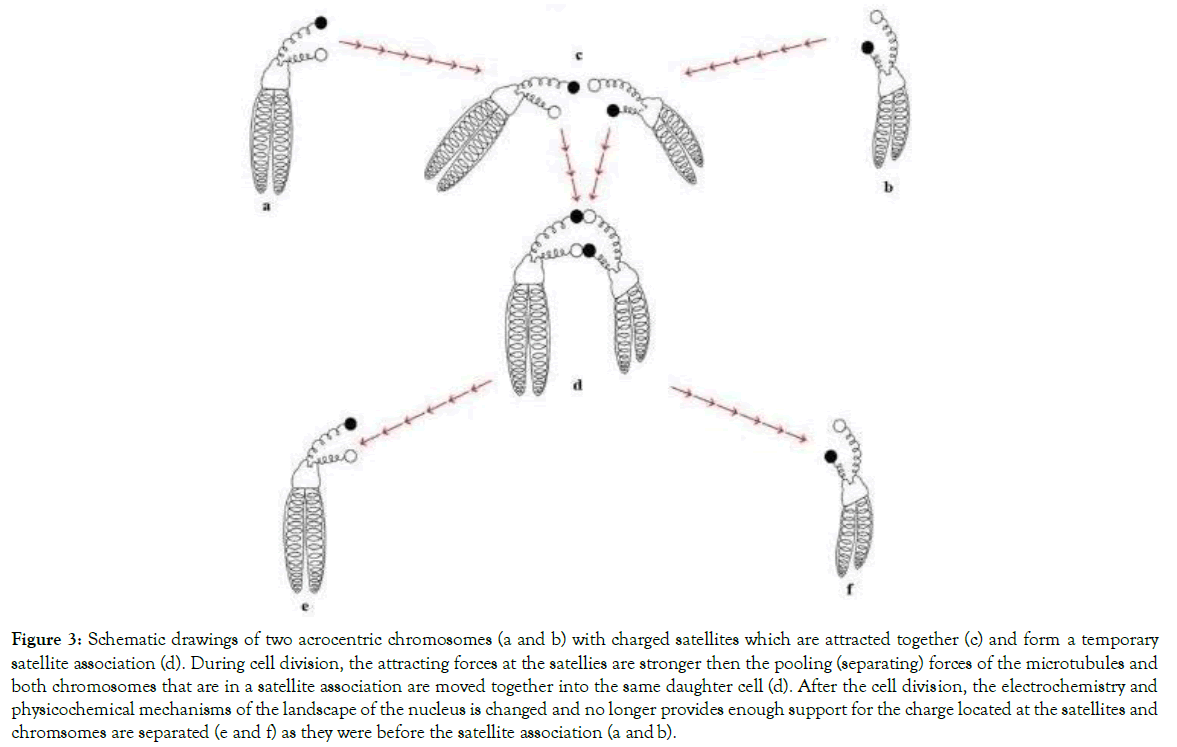
Figure 3. Schematic drawings of two acrocentric chromosomes (a and b) with charged satellites which are attracted together (c) and form a temporary satellite association (d). During cell division, the attracting forces at the satellies are stronger then the pooling (separating) forces of the microtubules and both chromosomes that are in a satellite association are moved together into the same daughter cell (d). After the cell division, the electrochemistry and physicochemical mechanisms of the landscape of the nucleus is changed and no longer provides enough support for the charge located at the satellites and chromsomes are separated (e and f) as they were before the satellite association (a and b).

Figure 4. Microphotographs of: abnormal female chromosome complement with three independent full copies of acrocentric chromosomes 21 known as T21 (DS) (a); abnormal male chromosome complement with three independent full copies of acrocentric chromosomes 13 known as T13 (PS) (b); and abnormal male chromosome complement with three independent full copies of acrocentric chromosomes 14 – T14.
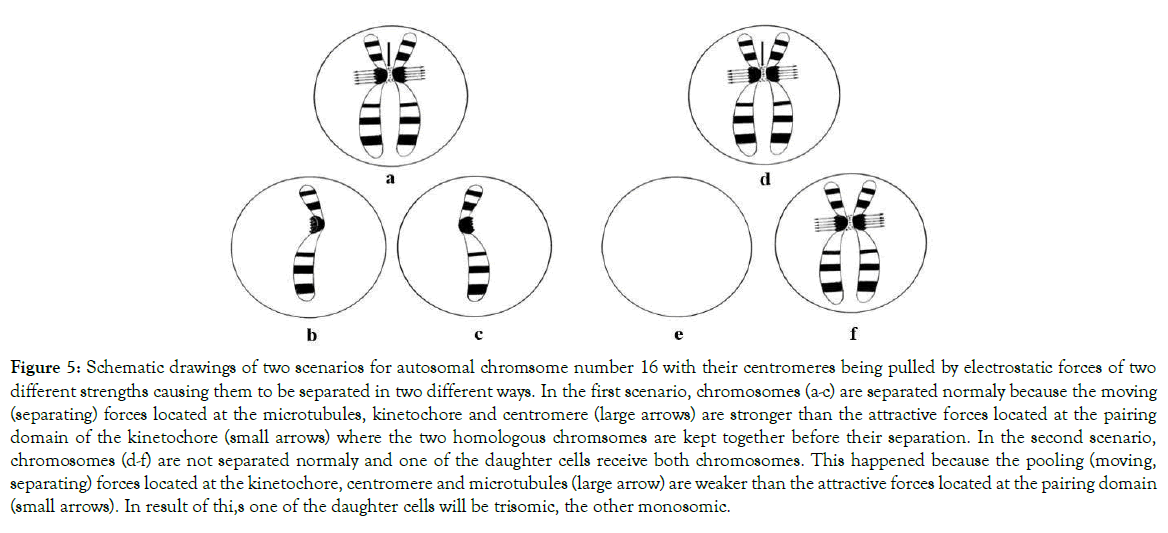
Figure 5. Schematic drawings of two scenarios for autosomal chromsome number 16 with their centromeres being pulled by electrostatic forces of two different strengths causing them to be separated in two different ways. In the first scenario, chromosomes (a-c) are separated normaly because the moving (separating) forces located at the microtubules, kinetochore and centromere (large arrows) are stronger than the attractive forces located at the pairing domain of the kinetochore (small arrows) where the two homologous chromsomes are kept together before their separation. In the second scenario, chromosomes (d-f) are not separated normaly and one of the daughter cells receive both chromosomes. This happened because the pooling (moving, separating) forces located at the kinetochore, centromere and microtubules (large arrow) are weaker than the attractive forces located at the pairing domain (small arrows). In result of thi,s one of the daughter cells will be trisomic, the other monosomic.

Figure 6. Microphotographs of: abnormal male chromosome complement with three copies of chromosome 16 (a); abnormal male chromosome complement with three copies of chromosome 18 known as ES (b); abnormal female chromosome complement with one copy of chromosome X, known as Monosomy X or TS (c); and abnormal female chromosome complements with three copies of chromosome X known as XXX syndrome (d).
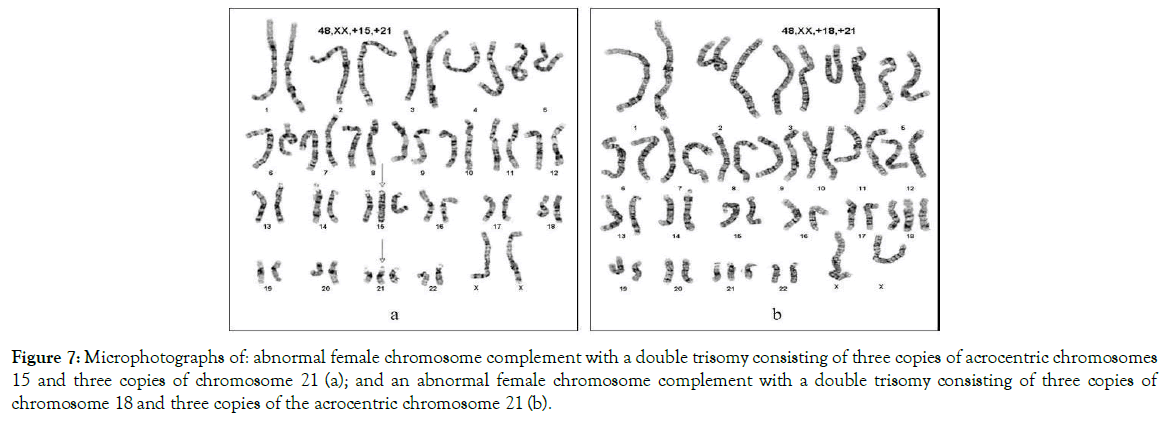
Figure 7. Microphotographs of: abnormal female chromosome complement with a double trisomy consisting of three copies of acrocentric chromosomes 15 and three copies of chromosome 21 (a); and an abnormal female chromosome complement with a double trisomy consisting of three copies of chromosome 18 and three copies of the acrocentric chromosome 21 (b).
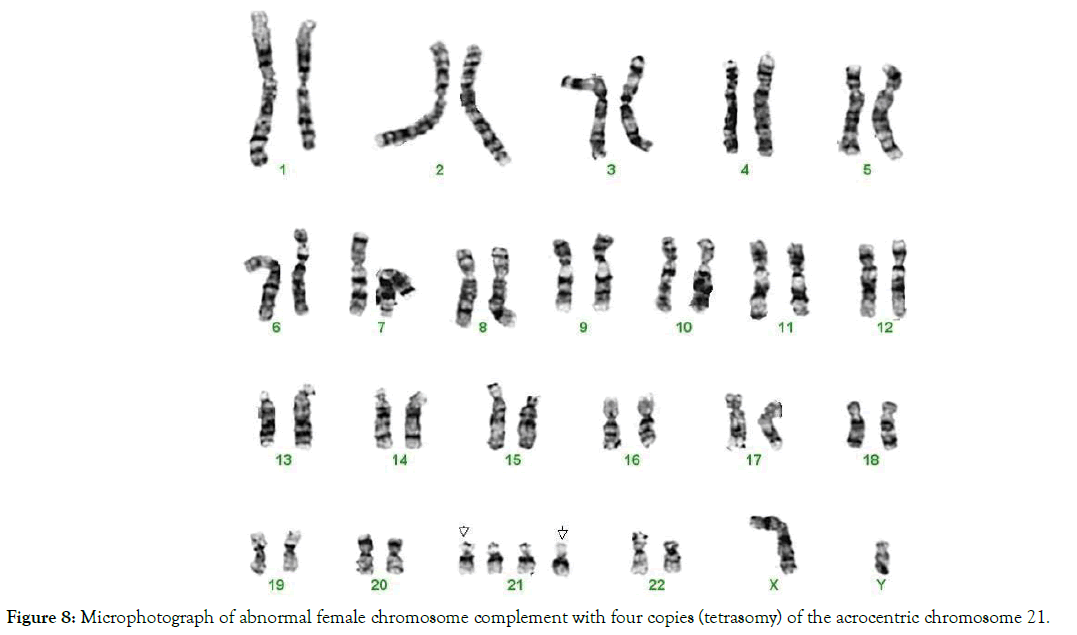
Figure 8. Microphotograph of abnormal female chromosome complement with four copies (tetrasomy) of the acrocentric chromosome 21.
Our long time clinical diagnostic studies with human chromosomes showed that both, centric fusion (Robertsonian) translocations and the supernumerary marker chromosomes, are caused by one and the same charge based mechanism as illustrated in Figure 9.
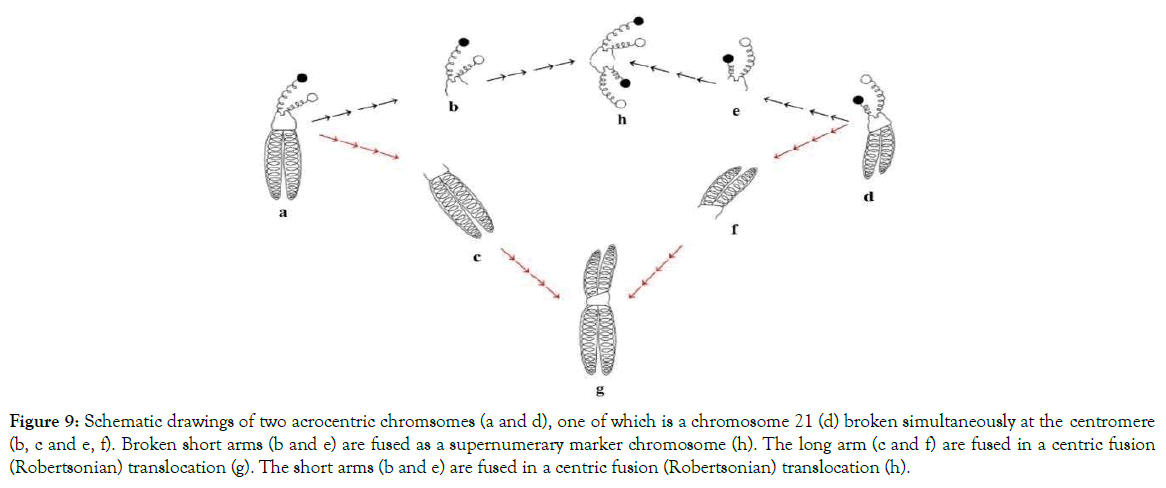
Figure 9. Schematic drawings of two acrocentric chromsomes (a and d), one of which is a chromosome 21 (d) broken simultaneously at the centromere (b, c and e, f). Broken short arms (b and e) are fused as a supernumerary marker chromosome (h). The long arm (c and f) are fused in a centric fusion (Robertsonian) translocation (g). The short arms (b and e) are fused in a centric fusion (Robertsonian) translocation (h).
Depending on the places where breaks, translocations and fusions of centromeres and adjacent areas occurred, different models of numerical abnormalities are shown in Figures 10-15. These figures provide an explanation for when, why, and how known variants of centric fusion numerical abnormalities occur including the mechanism responsible for the development of Robertsonian translocations with one centromere (monocentric) (Figure 15c); with two centromeres (dicentric) (Figures 11c and 12c); and no centromere (acentric – which are lost during the cells division because microtubules have no place to attach) (Figures 13c and 14c).
Figure 10. Schematic drawings of a smaller acrocentric chromosome from group G (a) and a larger chromosome from group D (b).
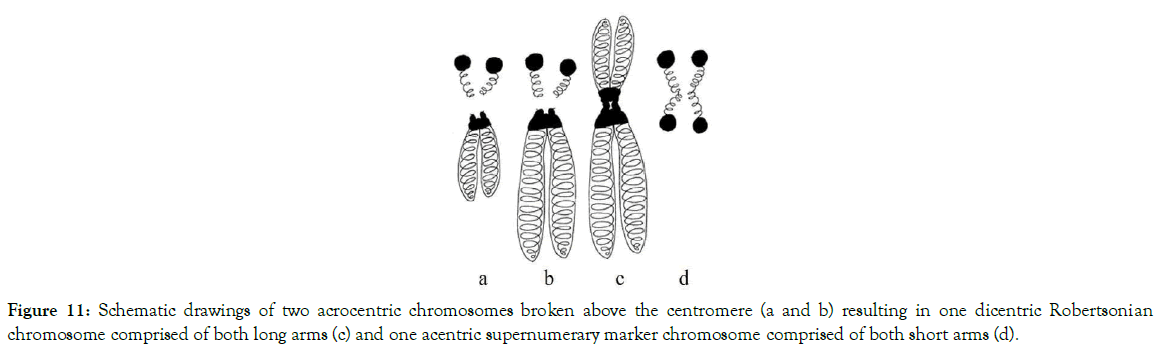
Figure 11. Schematic drawings of two acrocentric chromosomes broken above the centromere (a and b) resulting in one dicentric Robertsonian chromosome comprised of both long arms (c) and one acentric supernumerary marker chromosome comprised of both short arms (d).
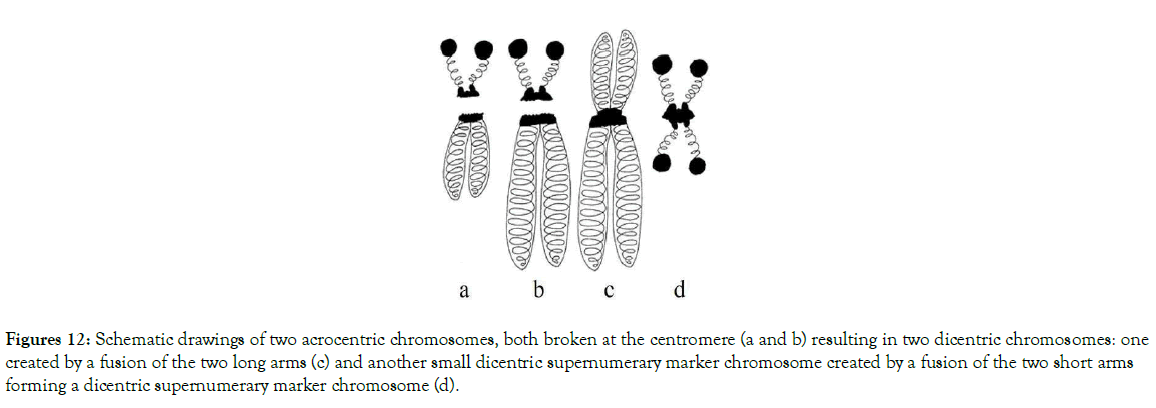
Figure 12. Schematic drawings of two acrocentric chromosomes, both broken at the centromere (a and b) resulting in two dicentric chromosomes: one created by a fusion of the two long arms (c) and another small dicentric supernumerary marker chromosome created by a fusion of the two short arms forming a dicentric supernumerary marker chromosome (d).
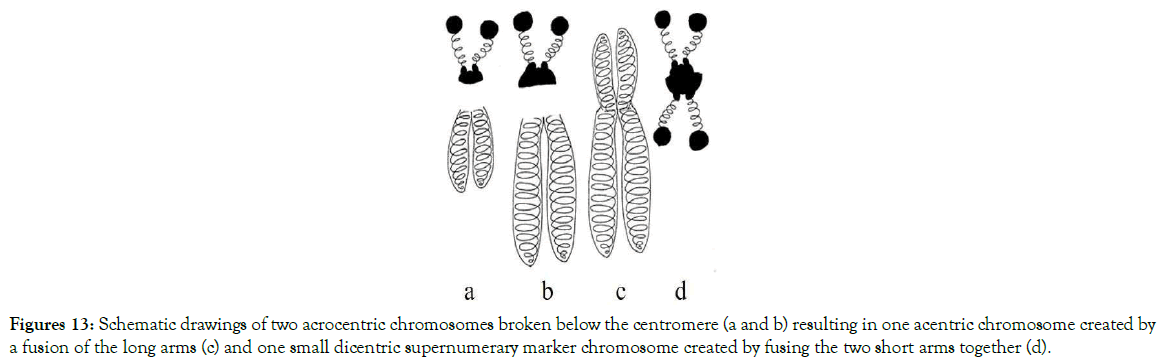
Figure 13. Schematic drawings of two acrocentric chromosomes broken below the centromere (a and b) resulting in one acentric chromosome created by a fusion of the long arms (c) and one small dicentric supernumerary marker chromosome created by fusing the two short arms together (d).
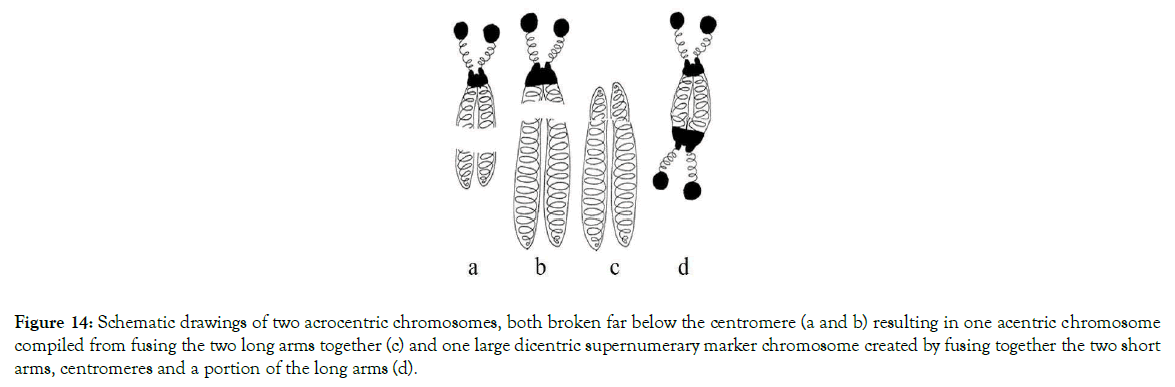
Figure 14. Schematic drawings of two acrocentric chromosomes, both broken far below the centromere (a and b) resulting in one acentric chromosome compiled from fusing the two long arms together (c) and one large dicentric supernumerary marker chromosome created by fusing together the two short arms, centromeres and a portion of the long arms (d).
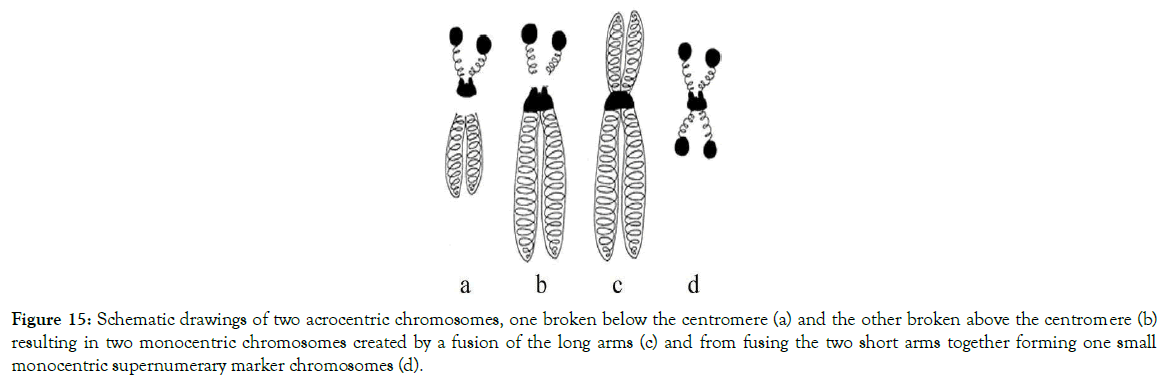
Figure 15. Schematic drawings of two acrocentric chromosomes, one broken below the centromere (a) and the other broken above the centromere (b) resulting in two monocentric chromosomes created by a fusion of the long arms (c) and from fusing the two short arms together forming one small monocentric supernumerary marker chromosomes (d).
In human karyotypes, centric fusion Robertsonian translocations of acrocentric chromosomes 13, 14, 15, 21 and 22 are found in 15 variants: five of these variants consist of the long arms of two chromosomes 13, 14, 15, 21 or 22 being fused (Figures 9 - 16); ten variants consist of the long arms of two different acrocentric chromosomes fused into a chromosome complement of 45 (Figure 16b) or 46 abnormal chromosome complement (Figure 16c).
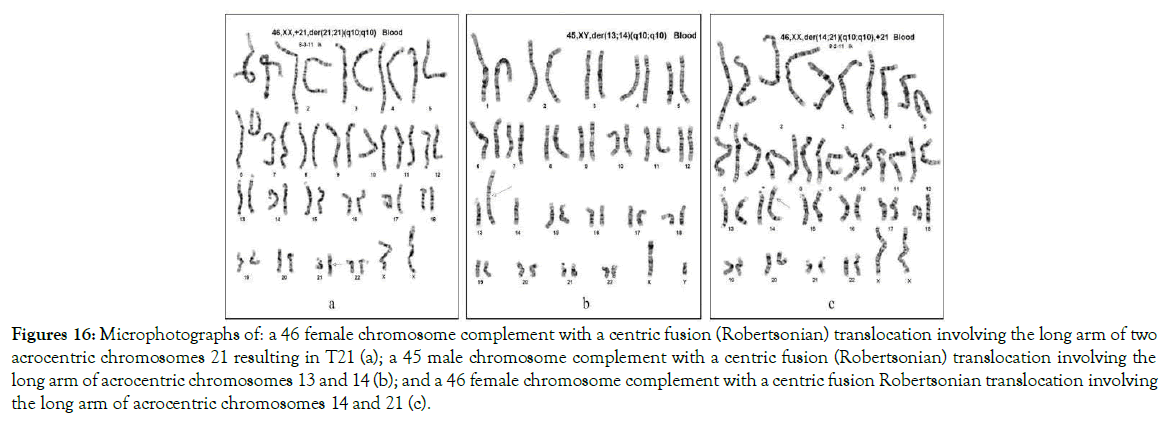
Figure 16. Microphotographs of: a 46 female chromosome complement with a centric fusion (Robertsonian) translocation involving the long arm of two acrocentric chromosomes 21 resulting in T21 (a); a 45 male chromosome complement with a centric fusion (Robertsonian) translocation involving the long arm of acrocentric chromosomes 13 and 14 (b); and a 46 female chromosome complement with a centric fusion Robertsonian translocation involving the long arm of acrocentric chromosomes 14 and 21 (c).
Supernumerary marker chromosomes caused by the same charge based fusions that are causing centric fusion (Robertsonian) translocation
Small (sSMC) and large (lSMC) supernumerary marker chromosomes were discovered relatively recently [39,40]. Our studies found that the supernumerary marker chromosomes are not isolated and different in origin as it was suggested so far. Our studies suggest that they developed under the influence of the same charge based mechanism that is responsible for the development of centric fusion Robertsonian translocations as described in Figures 10-15 resulting in the same dicentric, monocentric and acentric variants. Figure 17 presents two forms of supernumerary marker chromosomes including a dicentric sSMC and lSMC.
Trisomy caused by centric fusion (derivative) translocations of autosomal chromosomes
A trisomy caused from a derivative translocation develops from the same charge based mechanism that is responsible for the development of centric fusion Robertsonian trisomy and supernumerary marker chromosomes. The only difference is in the chromosomes that are fused. In this scenario, there are two autosomal chromosomes (Figure 18a) or one autosomal and one acrocentric (Figures 17- 22) chromosome.
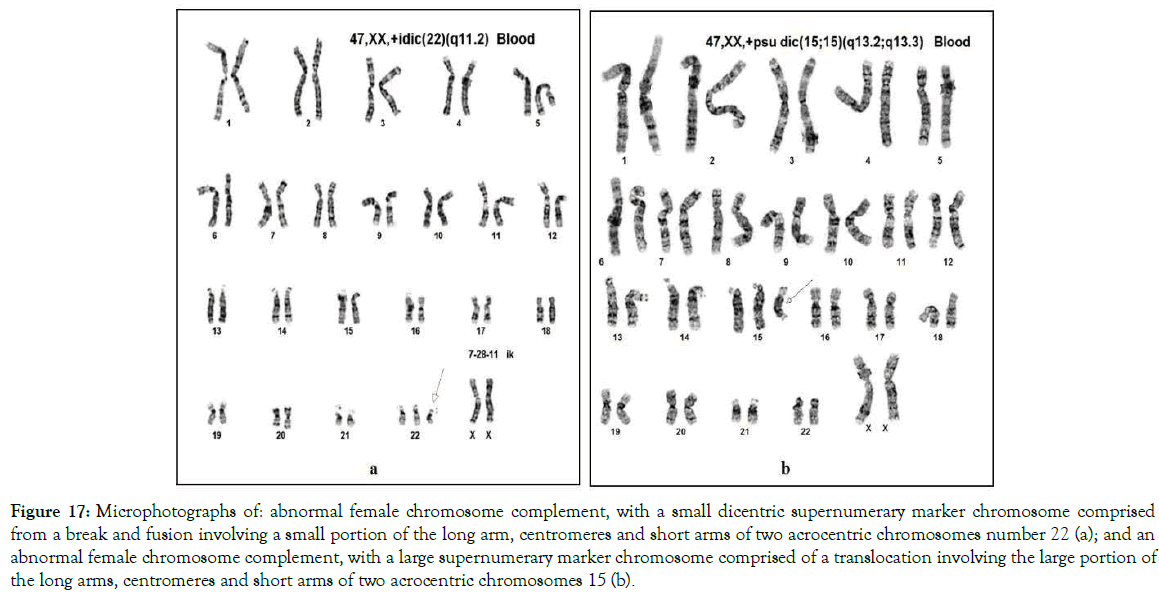
Figure 17. Microphotographs of: abnormal female chromosome complement, with a small dicentric supernumerary marker chromosome comprised from a break and fusion involving a small portion of the long arm, centromeres and short arms of two acrocentric chromosomes number 22 (a); and an abnormal female chromosome complement, with a large supernumerary marker chromosome comprised of a translocation involving the large portion of the long arms, centromeres and short arms of two acrocentric chromosomes 15 (b).
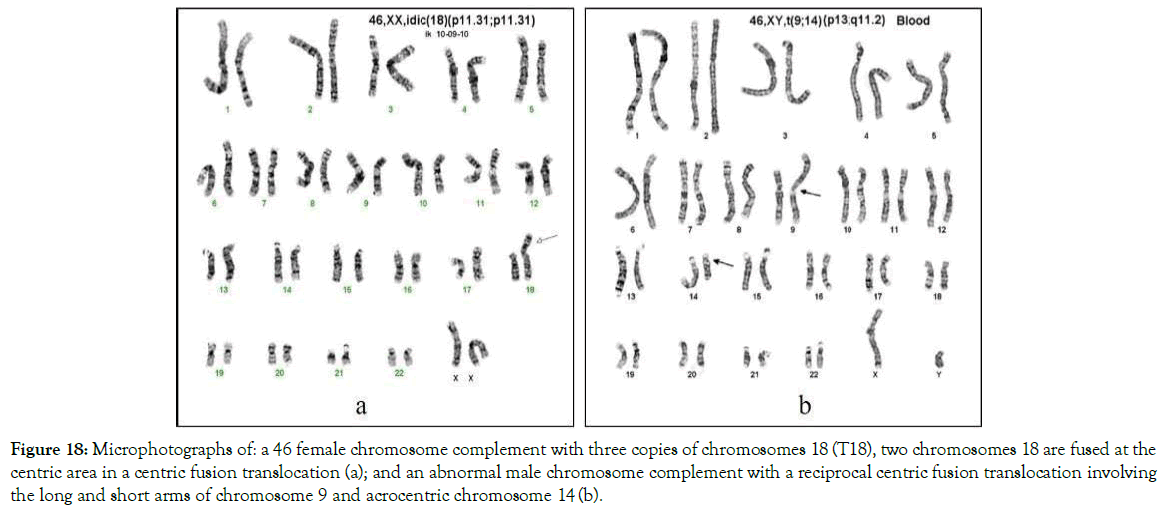
Figure 18. Microphotographs of: a 46 female chromosome complement with three copies of chromosomes 18 (T18), two chromosomes 18 are fused at the centric area in a centric fusion translocation (a); and an abnormal male chromosome complement with a reciprocal centric fusion translocation involving the long and short arms of chromosome 9 and acrocentric chromosome 14 (b).
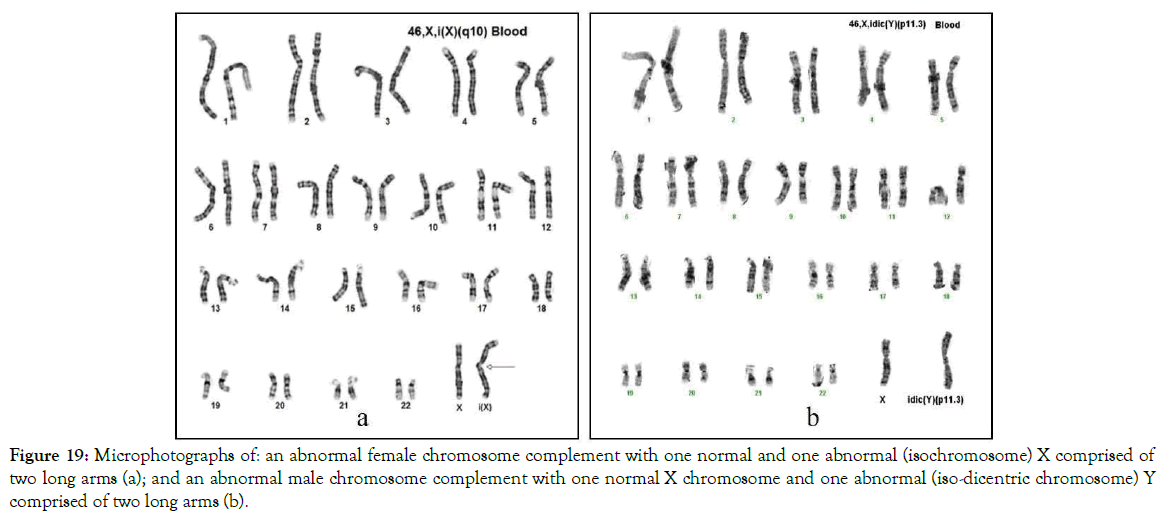
Figure 19. Microphotographs of: an abnormal female chromosome complement with one normal and one abnormal (isochromosome) X comprised of two long arms (a); and an abnormal male chromosome complement with one normal X chromosome and one abnormal (iso-dicentric chromosome) Y comprised of two long arms (b).
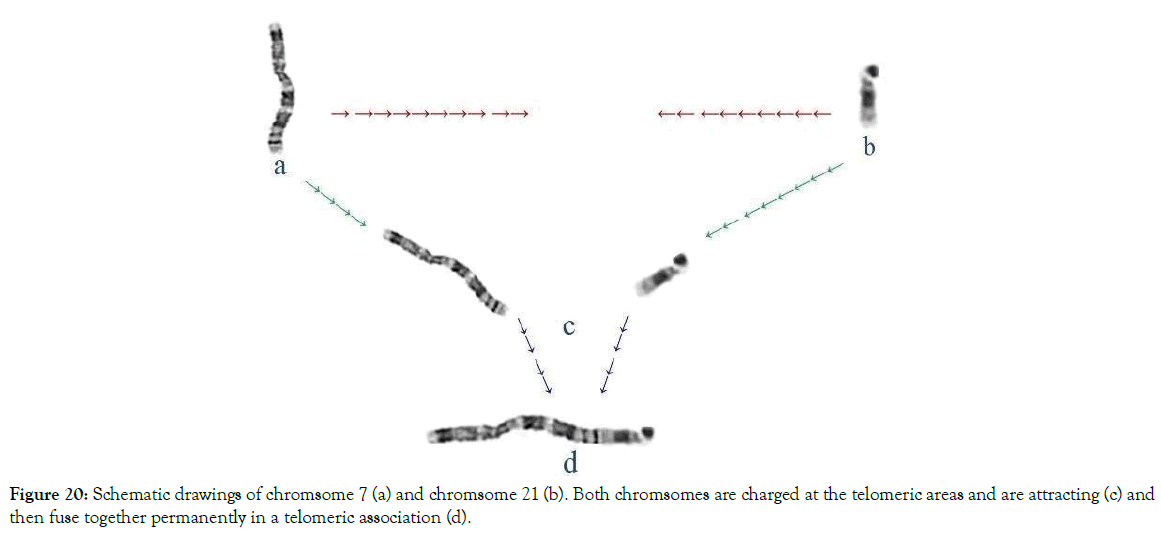
Figure 20. Schematic drawings of chromsome 7 (a) and chromsome 21 (b). Both chromsomes are charged at the telomeric areas and are attracting (c) and then fuse together permanently in a telomeric association (d).
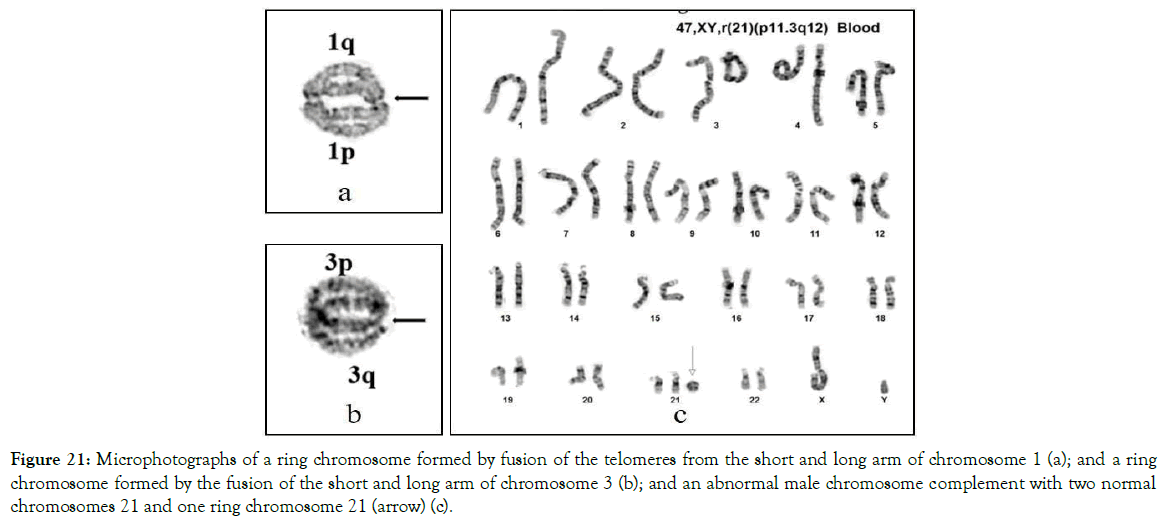
Figure 21. Microphotographs of a ring chromosome formed by fusion of the telomeres from the short and long arm of chromosome 1 (a); and a ring chromosome formed by the fusion of the short and long arm of chromosome 3 (b); and an abnormal male chromosome complement with two normal chromosomes 21 and one ring chromosome 21 (arrow) (c).
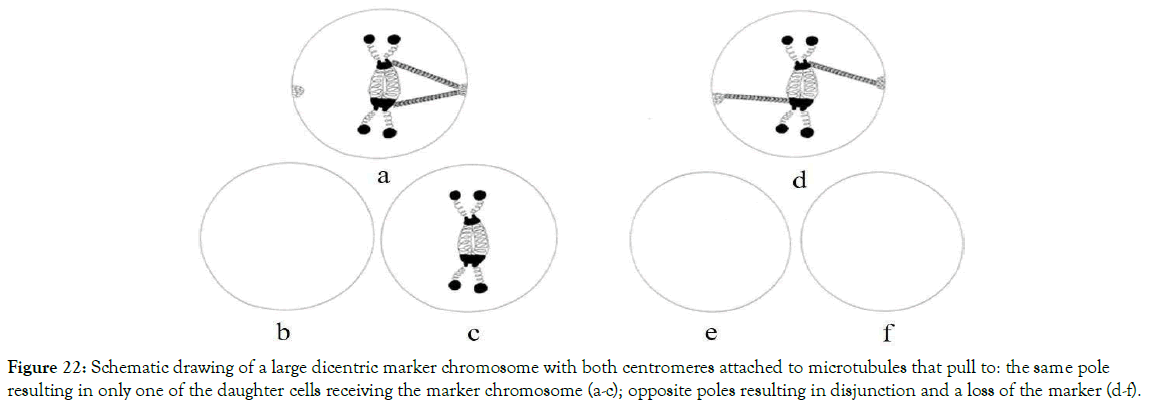
Figure 22. Schematic drawing of a large dicentric marker chromosome with both centromeres attached to microtubules that pull to: the same pole resulting in only one of the daughter cells receiving the marker chromosome (a-c); opposite poles resulting in disjunction and a loss of the marker (d-f).
Depending on the fused chromosomes and their combinations, these complements are described with different names of “derivative chromosomes”, “iso-chromosomes”, translocations, or ”centric fusion translocations”.
Trisomy caused by centric fusion (iso) translocations of sex chromosomes
Centromeres of sex chromosomes, like the centromeres of all chromosomes, are free of important genes, proteins and enzymes and therefore, they are broken and fused by the same charges that are available at the centromere. Our studies with centric fusion sex chromosomes are shown in Figure 19.
Trisomy caused by fusions in the form of telomeric associations
Our studies with human chromosomes showed that chromosomes 9, 12 and 14 are the most commonly found in telomeric translocations (Figure 20). The mechanism of this form of fusion is shown in Figure 21.
Trisomy caused by fusions known as ring formations
Ring chromosomes are known to exist primarily in acquired (cancer) abnormalities or after exposing chromosomes to X-rays. They are rarely found as constitutional abnormalities in plants, animals or humans. In our long-term studies with human chromosomes, constitutional ring chromosomes and trisomy caused by ring chromosomes were found in peripheral blood samples of patients with T21 [37] (Figure 22).
Mosaic monosomy, trisomy and supernumerary marker chromosomes caused by the difficulties of the spindle apparatus to segregate evenly
The first mosaic chromosomal abnormality was reported by Hamerton et al. in a mosaic form of T21 [41]. Today, the mosaic form of monosomy, trisomy and supernumerary marker chromosomes are found in chromosomes of plants, animals and humans [42].
Our studies suggest that mosaic forms of chromosomes are caused by one and the same charge mechanism. We suggest that these are caused by mechanical problems of the spindle apparatus which has failed to separate evenly into two daughter cells. Depending on the number and position of their centromeres and their attachment to the microtubules of the spindle apparatus, the results of these chromosomes could be: (i) incompatible for cell division and lost if they are acentric with no centromere and no possibility of microtubule attachment to the spindle apparatus; (ii) delivered to only one of the daughter cells due to the impossibility to send a single chromosome into two different cells; or (iii) tangled and lost if the microtubules of the spindle apparatus have complications with the attachment to the supernumerary chromosomes that are monocentric and have only one centromere or are large dicentric chromosomes possessing two centromeres located in different places as shown in Figure 22.
In every cell division, the number of abnormal daughter cells with a small or large supernumerary marker chromosome is reduced due to the increasing number of the new normal daughter cells. We know this fact from our long time clinical diagnostic studies of patients with T21 during their lifetime. The first study was done when the patient was a newborn where all of the studied cells (100%) were found to have a full T21. A study conducted seven years later revealed T21 in only 88% of his studied cells with the remaining 12% karyotypically normal. This study indicated that the full and mosaic form of T21 (DS) are not different but rather two chromosomal statuses of the same trisomy in transition.
Using charge of chromosomes we propose a solution for century old issues concerning Down, Turner, Patau and other syndromes caused by numerical abnormalities of chromosomes
In the long history of Down, Turner, Patau and other syndromes and health conditions there are several century old issues and questions that are not solved and not answered so far. Using charge of chromosomes we propose solutions and answers for six of them.
Why, from biological parents with normal phenotype and normal genotype of 46 chromosomes, are children born with an abnormal phenotype and abnormal genotype of 45 or 47 chromosomes?
This question is asked from the biological parents of children with Down, Turner, Patau and other syndromes caused by numerical abnormalities of chromosomes.
The answer is that numerical abnormalities of chromosomes are not caused by abnormal genes as it was suggested for many years. They are caused by the non-disjunctions or fusions of whole chromosomes that are exposed to strong, unbalanced, disturbing effects of electrical charge interactions. For the same reason, Down syndrome and other aneuploidies did not fit into the Law of inheritance discovered by Mendel [43-44]. According to this law, offspring inherited structural and functional characteristics which belong to their parents. This law did not apply to children with trisomies, monosomies and other aneuploidy. Offspring born with trisomies and monosomies possess new phenotypic characteristics which were not found in their parents, brothers, or sisters. Their abnormal chromosome number is also different from the normal chromosome complement of 46 chromosomes found in their biological parents and siblings.
Why are the same chromosomes that are shown to cause Down, Turner, Patau and other aneuploidy syndromes found as numerous variants of structural abnormalities?
This question is asked by those that study and analyze chromosomes for clinical diagnostic purposes in genetics.
The answer is because these numerical abnormalities of chromosomes are not caused by genes, proteins or enzymes. They are caused by charge based non-disjunctions or fusions that occur in different places of one or more chromosomes resulting in different combinations. For example, the non-disjunction of satellites illustrated in Figure 3 could cause five different variants of T21 known as: full T21 (Figure 4a); double trisomy with T21 and another acrocentric trisomy (Figure 7a); double trisomy with T21 and another autosomal trisomy (Figure 7b); and tetrasomy 21 (Figure 8). Ten more variants of T21 could develop by centric fusion (Robertsonian) translocations illustrated in Figure 3. Five of them developed when the long arm of one chromosome 21 is fused with the long arms of another chromosome 21 (Figure 16a); chromosome 13 (Figure 16b); chromosome 14 (Figure 16c), chromosome 15 or 22. Another five are variants with different numbers of centromeres known as dicentric (Figure 11 and 12), monocentric (Figure 15), and acentric (Figure 13 and 14) chromosomes. Three more variants of T21 are caused by telomeric associations (Figure 21), ring formation (Figure 22) and mosaic trisomy described in Section 2.7 and Figure 22. For this same reason, the excessive numbers of structural abnormalities and variants are also found in cases of monosomy X (TS), T13 (PS) and other numerical abnormalities of chromosomes.
Why offspring with one and the same numerical chromosome abnormality have different life expectancies?
It is unknown why, in some cases, an embryo with full T21 (DS) is not compatible with life and is spontaneously aborted, while in other cases, the embryo is born alive and lives a long life. Also, it is unknown why some individuals that are born with full T21 present with a range of health problems from mild to severe. The same problems and questions exist with patients diagnosed with TS, Klinefelter syndrome, and other aneuploidies. So far, these questions have not received complete answers.
The answer is because these numerical abnormalities are not caused by a single gene or protein as it has been suggested. They are caused by the electric charge effects that are causing not only non- disjunction or fusion resulting in T21 but they are also capable of causing micro-deletions, micro-duplications and micro-inversions found in the chromosomal complement of patients with T21 or other aneuploidy. These submicroscopic abnormalities were discovered in the last few years by utilizing molecular microarray and other higher resolution methods. Our comparative studies revealed that micro-deletions, micro-duplications and micro- inversions are found five, six, or even more times per patient when in conjunction with T21. Based on this fact, we suggest that some of the extremely variable clinical manifestations found in patients with T21, and other aneuploidies, could be explained by the occurrence of the charge-based micro-deletions, micro-duplications, micro- translocations and/or micro-inversions. Our understanding is that these miniscule abnormalities are perhaps capable of damaging important genes located in different chromosomes, which would cause additional complications, and affect the clinical aspects of patients with many forms of aneuploidies. For example, if these micro-abnormalities of a T21 individual are located in or near the genes that control the heart, they will have a negative influence leading to the development of ventricular septa defects or other heart diseases and syndromes. Those located in or near genes that are responsible for the development of the eyes, ears and other organs could lead to eye abnormalities, deafness, or other organ disorders which are common phenotypes of DS.
Why the correlation between the aneuploidy and heterochromatic variants of chromosomes was supported by some researchers while others doubted and rejected it?
Support was provided by researchers who were working with chromosomes in clinical diagnostic studies [26-28]. These researchers repeatedly reported that the heterochromatic regions were increased in size in patients with T21 and other aneuploidies. This was especially true for the heterochromatin on the satellites of acrocentric human chromosomes 13, 14, 15, 21, and 22. These satellites are often significantly increased in size and are found in satellite associations more often in patients with trisomy, monosomy and other numerical chromosomal abnormalities.
Based on these facts, Ohno et al. [27] and others suggested that there is a correlation between T21 (DS), monosomy X (TS) and other aneuploidies and the increased size and number of satellites and satellite associations.
Unfortunately, the majority of clinical specialists in genetics have doubted and rejected the existence of such a correlation because the satellites are built of heterochromatic areas which do not have any important protein and enzyme coding genes. Based on this fact, they argue that the increased size of satellites and the number of satellite associations are only coincidental and do not have anything to do with the mechanisms responsible for the development of aneuploidy.
The answer is in the purpose and the importance of heterochromatin which is not in the important genes which are missing. Our studies [23] revealed that the real purpose and importance of heterochromatin is to provide millions of positively charged histones and negatively charged DNA. Their interactions and electric charge effects plays an important role in many important constituents and events in the construction and function of chromosomes. Unfortunately, occasionally, they are responsible for the development of non-disjunctions and fusions resulting in trisomies, monosomies and other aneuploidy.
Why diagnosis and screening of T21 and other aneuploidy is so unpredictable due to false positive and false negative results?
The idea for screening and diagnosing new cases of DS during the early months of pregnancy belongs to Penrose [45]. He used the Advanced Maternal Age (AMA) notion which was based on the fact that every baby girl is born with a certain number of egg cells. The chromosomes in these egg cells remain “arrested” in the dicytotene stage until ovulation. This fact led to the development of two presumptions. First, that in an older mother, the “arrested” chromosomes spend more time in an inactive stage and therefore lose their flexibility and vitality. In turn, the second presumption was created that correlates chromosomes that have a low flexibility and vitality are at an increased risk for the development of DS and other aneuploidies.
The problem with this notion is that too many false negative and positive results have been obtained by the methods based on AMA. This problem became visible immediately after Penrose used AMA for the first time almost 90 years ago. Aware of this problem, Penrose and his team spent over 35 years studying AMA searching for an appropriate explanation in order to reduce the number of false negative and positive diagnoses [46-49]. Several modifications, including using alpha-fetoproteins from maternal serum along with the AMA were used but the problem was not solved and the number of the false results remained with the same high range.
This problem became larger and more prominent when the widely accepted predisposition of DS and aneuploidy based on AMA was put into question in the 1980’s. These questions occurred after extensive comparative studies with thousands of pregnant women of different ages were completed and compared by numerous research teams [50-52]. They concluded that pregnancies in women with advanced maternal age, who delivered in a modern tertiary care center, may be at no higher of a risk for adverse outcome than pregnancies in younger patients. This conclusion went even further when the mechanism for the formation of trisomies and its relationship to increased maternal age was declared to be not fully understood.
Of course, the AMA method has its merit in the development of aneuploidy as it has been shown that an older mother’s chromosomes do lose their flexibility, vitality and ability and have an increased risk with the development of trisomy or monosomy due to more probabilities of non-disjunction events. However, this risk is not equal for all mothers with advanced age. Over 17 years ago, we found an increased incidence of satellite charge and associations in both biological parents of a baby with T21 [37]. At this period of time, we were not able to provide an appropriate explanation and we presented these findings as “a rare form of DS with unusual parental cytogenetic characteristics”. Years later, our studies show that the reasonable explanation is hidden in the electric charge effects of chromosomes which were not studied and remained unknown in clinical genetics where AMA is used for screening and predicting of T21 and aneuploidy.
Our understanding is that mothers of any age are at an increased risk to have a baby with trisomy or monosomy if they have in their chromosome complement strong unbalanced charge effects. Conversely, mothers of any age are at low risk to have a baby with trisomy or monosomy if they have balanced charge interactions in their chromosome complements. We suggest that the screening and predisposition of DS and other aneuploidies should be more exact with decreased false negative and positive results if the screening is based not only on the AMA but also on the electric charge effects and properties of chromosomes of the biological parents.
Why all programs and efforts for prevention of Down syndrome and aneuploidy have failed?
Discovered by Martha Gautier and reported by Lejeune [53] more than 60 years ago, T21 is known to be the most commonly distributed chromosomal abnormality in humans [1]. Depending on the geographical regions and other factors, the incidence is approximately one in every 650 – 800 live births. This distribution rate is reported in every reference book of human syndromes and inherited disorders [2]. Attempts to establish control and reduce the incidence rate of T21 have been made by the foundations for DS which have been organized at the private, state, federal, national and international level. One of the primary aims of these programs has been to fully sequence human chromosome 21 [6] with suggested implications for a better understanding and possible control over the distribution of DS. Unfortunately, none of these studies were able to establish the expected control on the distribution of T21. Today, DS continues to have an incidence rate of 1: 650 - 800 as it was before.
The same problems exist with the distribution and control of all other aneuploidies [2]. These problems indicate that the widely used methods for prevention and control of DS and aneuploidy do not work. Our understanding is that it is impossible to have control and to reduce the distribution of DS and other aneuploidies that are caused by the electric charge effects of chromosomes by studying only genes and proteins. Their distribution could be controlled and reduced only when the electric charge effects that are responsible for their development are fully characterized, widely used and accepted in clinical genetics in conjunction with classical chemical and biochemical theories.
Using charge of chromosomes we proposed solutions for issues concerning health conditions caused by numerical abnormalities of chromosomes
These are the same numerical abnormalities that are causing DS, TS, PS and other aneuploidy. They are also causing infertility, spontaneous abortions, mental and physical disabilities and other health conditions that have a chromosomal etiology. According to some authors [1], Trisomy 16 and 18 are found to be the most common chromosomal abnormalities in spontaneous abortions while Trisomy 21 and Monosomy X are considered to be the main sources for a wide range of mental and physical disabilities. The main problem in these studies was in the exact mechanism which is responsible for the development of chromosomal abnormalities that cause infertility, spontaneous abortions, intellectual and physical disability. The mechanism was not fully understood and for this reason it is still difficult to predict and prevent spontaneous abortions, intellectual and physical disabilities that are caused by chromosomal abnormalities.
Our proposal for a solution is based on the same charge based mechanisms that are proposed to solve issues concerning DS, TS, PS and other syndromes and aneuploidy described above.
Using a new approach and long-term studies, we developed novel ideas and proposed a charge based mechanism responsible for the development of non-disjunction and fusion of whole or broken chromosomes which are resulting in monosomy, trisomy and other numerical abnormalities of chromosomes known as aneuploidy. We proposed novel ideas, approaches, and strategies for better understanding, diagnosing, and screening and in turn, curing and preventing DS, TS, PS and other syndromes and health conditions caused by numerical abnormalities of chromosomes using electrical charge based theories.
The novel charge based ideas, approaches, and strategies described in this publication are presented as an alternative from the previously widely accepted ideas, approaches and strategies for gene-protein-enzyme mechanism responsible for the development of numerical abnormalities of chromosomes and their implications in human health conditions including DS, TS, PS and other syndromes with a chromosomal etiology.
This study was supported by internal cytogenetic research and development funds from the Human Genetics Laboratory, Munroe-Meyer Institute for Rehabilitation and Genetics, University of Nebraska Medical Center, USA. This publication is dedicated to the loving memory of Prof. Warren Sanger, a founder and over 40 years director of the Human Genetics Laboratory, extraordinary administrator, investigator, colleague, author, and friend, in respect of his participation and strong professional support given to this study.
Not applicable.
The authors declare that they have no competing interests.
No funding for this research.
Citation: Kanev I, Grove J, Novak K (2020), Numerical Abnormalities of Chromosomes Caused by the Electric Charges and Their Implications on Down, Turner, Patau and Other Human Syndromes and Aneuploidies. J Down Syndr Chr Abnorm 6:132. doi: 10.4172/2472-1115.20.6.132
Received: 18-Apr-2020 Accepted: 08-Jun-2020 Published: 11-Jul-2020 , DOI: 10.35248/2472-1115.20.6.132
Copyright: © 2020 Kanev I, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited