Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2016) Volume 5, Issue 4
We used ICP mass spectrometry and gas chromatography to examine the nutritional properties of the flower organs of saffron crocus grown in two cultivation fields. The pollenkitt consisted of a variety of mainly unsaturated fatty acids of dietary value, and fats of the same type were also on the anther wall. Microelements important for the human body were at concentrations allowable in both pollen and anther wall cells. Lead and mercury were below allowable levels, unlike arsenic which in pollen of the Field A was 1.70 μg/g, and cadmium which in the Field B was 0.29 μg/g in pollen and 0.43 μg/g in the anther wall cells. The contamination of the stamens was associated with the high concentration of heavy metals found in the soil. Phosphorus, magnesium and calcium were high and sodium low in both pollen and anther wall cells, regardless of the cultivation fields. Styles and corms were rich in macroelements and not contamined by heavy metals. If on one hand pollen and anthers of saffron deserve attention as dietary supplements, on the other it must be taken into due account their tendency to absorb toxic metals from the soil.
Keywords: Pollenkitt fats; Minerals; Pollen; Anter wall; Styles; Corms; Crocus sativus L
Saffron crocus (Crocus sativus L.) belonging to the family Iridaceae, is a perennial autumn flowering plant unknown in the wild and widely cultivated for its flowers with trilobal scarlet styles from which it derives the saffron spice well known around the world for its aromatic and medical properties [1]. Its pollen is sticky because of a pollenkitt layer that the nurse cells (tapetum) of the anthers deposited on the pollen exine. Pollenkitt is a complex mixture composed mainly of fatty acids, whose composition is little known [2,3], which performs many functions in the pollen [4] including that of providing a food reward for pollinators. In addition to pollenkitt pollen is a source of nutrients that vary depending on the species and habitats [5-7], most of the data relate to the pollen collected by bees which is considered as a full reserve of nutrients for the human body [7,8]. A typical food supplement based on pollen and used by humans is the royal jelly, the food reserved for the queen bee. Also there is a widespread medicinal use of pollen supported by pharmacological studies in rats and rabbits, as well as clinical tests, which suggest benefits for the immune system, nervous, circulatory and urinary [9,10]. On the contrary, studies on the composition and use of the anthers are missing. Also there is no specific legislation on the quality of the pollen, so that less attention is paid to pollen contamination, in particular from the metals that accumulate in soils exposed to industrial and intensive agricultural activities.
This study is part of a research project on saffron investigating the nutrients and soil pollutants accumulated in the flowers and bulbs, with the aim of safeguarding the quality of the drug, as well as to retrieve the parts discarded by the saffron producers for use as food supplements.
Flowers and corms of saffron crocus (C. sativus L.) were taken from two rural fields of saffron cultivation: Field A of large extension located 40 km South-East of L’Aquila (Italy), and the Field B of small extension located 15 km East of L’Aquila. The samples consisted of: 1) pollen separated with a brush from opened anthers, 2) anthers without pollen (anther wall), 3) commercial dried styles, 4) corms during the vegetative stage that follows the flowering, and 5) soil around the corms. Pollen and anthers were air-dried at room temperature. Each test for the analyses were in triplicate.
Fatty acids
Extraction of fatty acids and methyl ester preparation: 0.9 g of each sample consisting of dried pollen or dried anthers without pollen, were treated with 30 ml of n-hexane (Carlo Erba Reagents) for 4 min at room temperature to remove pollenkitt. The solution was filtered on paper filter 40 W and brought to dryness with a Rotavapor (Büchi). The residue was taken up with 2 ml of heptane (Carlo Erba Reagents) and then added to 0.2 ml of 2N potassium hydroxide in methanol solution (methyl esters preparation). After agitation for 30 sec the solution was centrifuged (ALC 4235) for 4 min at 3500 rpm. Approximately 0.2 ml of supernatant was diluted with 1 ml of heptane to obtain the solution to inject into the gas chromatograph.
Gas chromatography: The fatty-acid composition was determined using a gas chromatography system (HRGC 8000 Series, Fisons Instruments, Milano, Italy) equipped with an SPTM-2380 (Supelco, Bellefonte, PA, USA) fused silica capillary column (60 m × 0.32 mm ID × 0.2 μm film thickness). The oven temperature programme was from 70°C to 220°C at 2°C/min and held at 220°C for 20 min, and then from 220°C to 240°C at 2°C/min and held at 240°C for 10 min. The detector temperature was 260°C. Hydrogen was used as the carrier gas with a flow of 1.2 ml/min. The samples (0.4 μl) were applied by on-column injection. All the analyses were in duplicate for each sample.
For qualitative analysis a fatty acid methyl ester (FAME) mixture (SupelcoTM 37 components FAME Mix, Bellefonte, PA, USA) was preliminarily injected into the column to compare their retention times.
For quantitative analysis the following formula was applied to calculate the percentage of each fatty acid: %Fatty acid=Ax × 100/ΣA, where Ax is the area under the peak corresponding to the fatty acid x; and ΣA is the sum of the areas under all the peaks.
Micro-macroelements
Mineralization and analysis of the samples: 0.5-1 g of each dried sample were treated for 120 min at 95°C on a plate mineralizing (DigiPrep, SCP Science, Canada) with a high purity solution consisting of: a) 3 ml H2O2 (30% v/v) (Carlo Erba), b) 3 ml HNO3 (69%) (Panreac, Spain), and 9 ml HCl (5%) (Panreac, Spain). After cooling the mineralized solution was diluted with distilled water (Carlo Erba) to 50 ml and then filtered with vacuum filters (DigiFilters SCP Science, Canada). The samples were analyzed by the Optima 8300 ICP Spectrometer PerkinElmer equipped with a cyclonic chamber injector. Antimony, Mercury and Selenium were analyzed with the sodium borohydride (NaBH4) reducing method by using the above ICP spectrometer. Cadmium and Barium were analyzed by atomic absorption spectrometry (AAnalyst 800 PerkinElmer) equipped with graphite furnace (GFAA).
For both ICP-OES and GFAA analyses, a certified NIST standard containing all the analytes was used (criterion of acceptability: ± 10% on the value of the certificate standard). For analyses with GFAA, the analyte characteristic mass were also checked (criterion of acceptability: ± 20%).
The final concentration of each analyte was calculated with the formula:
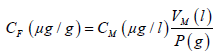
CF: final concentration of the analyte, CM: concentration of the analyte in the mineralized material, VM: final volume of the sample mineralized, P: weight of the sample.
Fatty acids in pollenkitt
Pollen: As shown in Table 1 the pollenkitt of pollen consisred of a variety of fatty acids (28 types) mostly with long chain (C18=56.78%) and with a prevalence of polyunsaturated fatty acids (55.82%), the ratio between the unsaturated and saturated was 2.2. Both fatty acids that are recurrent in plants such as palmitic, oleic, stearic, behenic, and those sporadic such as myristoleic, butyric and vaccenic acid, were present. The most common unsaturated were those considered essential for the human body, such as linolenic (35.26%) and linoleic acid (13.45%), which are the precursors of ω-3 and ω-6 known as lipid-lowering factors for the human organism [11]. Of interest, despite with low values, the presence of DPA (2.32%), DHA (2.60%) and EPA (0.48%), the typical precursors of eicosanoids such as prostaglandins of series 1 and 3, and leukotrienes that are messengers of hormonal type known for their antioxidant properties, vasodilatory and neuroprotective [12]. These Ω-3 acids are typically produced by algae and abundantly present in the oil of fishes that feed on algae, they are reported in very few plants [13], and in the seeds but not in pollen. The moinsatured class ω-9 was significantly present in pollenkitt with the oleic (5.59%), the acid typical of olive oil, and the eicosenoic (4.97%) typical of rapeseed oil.
| Fatty acids % | Pollenkitt | SDa | Anther wall | SDa | |
|---|---|---|---|---|---|
| C4:0 | Butyric | 0.88 | 0.04 | 4.06 | 1.15 |
| C6:0 | Capronic | 0.22 | 0.04 | 1.24 | 0.19 |
| C8:0 | Caprylic | 0.09 | 0.00 | 0.09 | 0.05 |
| C10:0 | Caprinic | 0.17 | 0.01 | 0.00 | 0.00 |
| C11:0 | Undecanoic | 0.02 | 0.01 | 0.00 | 0.00 |
| C12:0 | Lauric | 8.73 | 0.02 | 0.48 | 0.65 |
| C13:0 | Tridecanoic | 0.02 | 0.00 | 0.07 | 0.00 |
| C14:0 | Myristic | 1.35 | 0.00 | 0.34 | 0.02 |
| C14:1 | Myristoleic | 1.44 | 0.12 | 1.22 | 0.32 |
| C15:0 | Pentadecanoic | 0.08 | 0.00 | 0.00 | 0.00 |
| C16:0 | Palmitic | 15.27 | 0.08 | 10.23 | 2.67 |
| C16:1 | Palmitoleic | 0.12 | 0.01 | 0.11 | 0.07 |
| C17:0 | Heptadecanoic | 0.11 | 0.01 | 0.00 | 0.00 |
| C17:1 | Heptadecenoic | 0.04 | 0.02 | 0.08 | 0.00 |
| C18:0 | Stearic | 1.86 | 0.01 | 0.56 | 0.09 |
| C18:1 | Oleic | 5.59 | 0.01 | 6.08 | 1.54 |
| C18:1 | Vaccenic | 0.62 | 0.01 | 0.93 | 0.20 |
| C18:2 | Linoleic | 13.45 | 0.13 | 15.14 | 1.23 |
| C20:0 | Arachic | 0.19 | 0.01 | 0.19 | 0.00 |
| C18:3 | Linolenic | 35.26 | 0.25 | 35.00 | 2.54 |
| C20:1 | Eicosenoic | 4.97 | 0.03 | 5.53 | 1.11 |
| C20:2 | Eicosadienoic | 1.71 | 0.11 | 2.35 | 0.78 |
| C22:0 | Behenic | 1.83 | 0.12 | 1.93 | 0.88 |
| C22:1 | Erucic | 0.42 | 0.07 | 0.43 | 0.06 |
| C24:0 | Lignoceric | 0.23 | 0.01 | 0.23 | 0.00 |
| C20:5 | Eicosapentaenoic | 0.48 | 0.03 | 1.19 | 0.90 |
| C22:5 | Docosapentaenoic (DPA) | 2.32 | 0.06 | 3.60 | 1.20 |
| C22:6 | Docosahexaenoic (DHA) | 2.60 | 0.33 | 8.92 | 0.66 |
| SFAsb | 31.01 | 19.42 | |||
| MUFAsc | 13.18 | 14.38 | |||
| PUFAsd | 55.82 | 66.20 |
Table 1: Fatty acids % in pollenkitt and on the anther wall of saffron crocus Field A.
Unsaturated fatty acids consisted mainly of palmitic acid (15.27%) and lauric (8.73%). The first typical of oil palm which is used as an ingredient in many foods, is recurrent in both animals and plants, it is a main precursor of the wax esters that bees produce to build the cells of the honeycomb [14]. The second that is recurrent in tropical oils (coconut and palm) has antiseptic properties [15], to counteract biotic contaminations of the pollen.
The above data referring only to the pollenkitt fats are partially comparable with those of the literature that report the total fats of pollen including those cytoplasmic The comparison, however, shows a greater variety of fatty acids in saffron crocus, with relatively higher percentage of unsaturated, these in various types of pollen collected by bees do not exceed 66% and the corresponding ratio unsaturated/ saturated never reachs the values we found in saffron crocus [6,16-18].
Anther wall: The anther wall after dehiscence and pollen dispersal is reduced to epidermis and endothecium [19]. The majority of the fatty acids that we removed from the anthers with n-hexane were presumably the remains of pollenkitt precursors present on endothecium after anther dehiscence. They consisted of a variety of fatty acids (Table 1) similar to that of the pollenkitt from exine, but the rate of unsaturated acids was higher than in pollenkitt, so the ratio between unsaturated (80.58%) and saturated (19.42) was very high (4.1). Of the saturated, some were more abundant than in pollenkitt as butyric acid, well known as a typical component of rancid butter and hard cheese from which emanates a pungent odor, others such as lauric and palmitic acids were less present than in pollenkitt. In the literature we did not find any data about fatty acids on the anther wall.
Micro-macroelements
Pollen and anther cells from fields A and B: The concentrations of micro -and macro elements in pollen and the cells of the anther wall were reported in Table 2. Micronutrients well known as components of many proteins and enzymes were at low concentrations in both pollen and anther cells, regardless of the growing fields. Among these, copper, zinc and manganese which are needed in small daily doses for the human body, and the iron that is essential in higher daily doses of the order of 10 mg. Some deleterious trace elements, such as lead and mercury were not found, or they were at low concentrations in pollen and anthers from both fields. Diversely, there was variability concerning arsenic and cadmium content depending on the cultivation fields. Arsenic varied in pollen from <0.50 μg/g (Field B) to 1.75 μg/g 8 (Field A), cadmium from <0.05 (Field A) to 0.29 μg/g (Field B) as regards pollen and from <0.05 to 0.43 μg/g as concerns the anthers. With reference to the limits imposed by the legislation for food, European Commission to amend Regulation EEC No. 1881/2006 and with effect from 01.01.2015, has set for cadmium a limit ranging from 0.05 to 0.20 mg/kg wet weight for food plants. Since our present data are based on dry samples, they could be considered borderline, at least for the pollen. However, in the absence of specific legislation on pollen, some authors [5] propose for cadmium, arsenic, lead and mercury the limit of 0.1 μg/g, 0.5 μg/g, 0.5 μg/g and 0.03 μg/g respectively, for optimum pollen quality. Contamination with arsenic and even more with cadmium, has been reported in bee pollen from several countries (Europe, China, Brazil), and the present study is a new alert for pollen from rural fields. For arsenic high concentrations were found in Brazil [20,21], with values ranging 0.01 to 2.65 μg/g, China [6], with 5.24 μg/g, and in Serbia with 0.104 to 5.573 μg/g [22], and 1.090 to 9.640 μg/g [23]. For cadmium, high values were found in Poland with 0.48 to 0.66 μg/g [24], and even from 45.6 to 92.0 μg/g [25].
| Micro-macroelements | Pollen Field A |
RSDa % |
Pollen Field B |
RSDa % |
Anther cells Field A |
RSDa % |
Anther cells Field B |
RSDa % |
|---|---|---|---|---|---|---|---|---|
| Aluminum | 33.00 | 1.02 | <2.00 | 32.00 | 0.95 | <1.00 | ||
| Antimony | <b0.05 | <0.08 | <0.05 | <0.05 | ||||
| Arsenic | 1.70 | 1.12 | <0.80 | 1.72 | 1.20 | <0.50 | ||
| Cadmium | <0.05 | 0.29 | 0.33 | <0.05 | 0.43 | 0.79 | ||
| Cobalt | <0.20 | <0.50 | <0.20 | <0.30 | ||||
| Chrome | <0.20 | <0.50 | <0.30 | <0.30 | ||||
| Iron | 60.00 | 0.09 | 28.37 | 2.03 | 70.90 | 0.95 | 68.90 | 1.55 |
| Manganese | 32.70 | 0.77 | 42.11 | 0.77 | 30.20 | 0.70 | 32.20 | 0.74 |
| Mercury | <0.05 | <0.08 | <0.05 | <0.05 | ||||
| Nickel | <0.50 | <0.80 | 0.71 | 1.10 | 0.91 | 1.00 | ||
| Lead | <0.20 | <0.40 | <0.40 | <0.20 | ||||
| Copper | 19.00 | 1.13 | 18.40 | 1.27 | 18.90 | 1.67 | 18.00 | 1.87 |
| Selenium | <0.20 | <0.20 | <0.10 | <0.10 | ||||
| Vanadium | <0.50 | <0.80 | <0.50 | <0.50 | ||||
| Zinc | 51.00 | 0.97 | 54.15 | 2.04 | 42.80 | 2.00 | 40.10 | 2.12 |
| Tin | 1.10 | 1.52 | n.d.c | 0.99 | 1.40 | n.d.c | ||
| Barium | <0.80 | <0.50 | <0.80 | <0.50 | <0.50 | |||
| Magnesium | 1304. | 3.16 | 1200. | 0.44 | 1820. | 0.19 | 1800. | 0.13 |
| Calcium | 612. | 1.17 | 760. | 0.46 | 1030. | 0.41 | 1030. | 0.29 |
| Sodium | 30.00 | 0.97 | 40.00. | 0.12 | 30.00 | 1.29 | 30.00 | 0.99 |
| Phosphorus | 4466. | 3.14 | 5330. | 0.21 | 4400. | 0.60 | 5250. | 0.67 |
Table 2: Micro-macroelements (μg/g) in pollen grains and cells of the anther wall of saffron crocus of the Fields A and B.
Macroelements, with the exception of sodium, were present in our samples in relatively large amounts both in pollen and anther cells, some differences were found for magnesium and calcium which were more concentrated in the anthers. In the literature there are data of the saffron pollen from Iran cultivations [26], relatively to zinc, iron, copper, manganese, magnesium, sodium, calcium and potassium. From the comparison with our data, it emerges that the concentrations in the pollen of Iran are higher in particular for iron (194 ppm), magnesium (3357 ppm) and even more for sodium (1100 ppm). For sodium, concentrations much higher than in the present pollen are recurrent also in other plant species [6,16]. The concentrations of micro-macroelements in both pollen and the anther cells did not change following our treatment with solvents to remove pollenkitt, the data were similar to those in the Table 2 and are not reported.
Styles from fields A and B: As reported in Table 3 the styles of the two fields showed similar amounts of micro-elements except for iron which was more concentrated in stigmas of the Field A. Heavy metals were at low concentration and even cadmium and arsenic that in pollen exceed the recommended level [5]. For the macro-elements the major differences were for Magnesium, Calcium and Sodium which were more concentrated in styles of the Field B.
| Field A | RSD%a | Field B | RSD%a | |
|---|---|---|---|---|
| Aluminum | 5.01 | 0.71 | 5.29 | 0.96 |
| Arsenic | <b0.50 | <0.50 | ||
| Beryllium | 0.59 | 1.03 | 0.59 | 1.03 |
| Cadmium | 0.15 | 1.12 | 0.11 | 1.16 |
| Cobalt | 0.49 | 0.73 | 0.48 | 0.88 |
| Chrome | 0.49 | 0.58 | 0.49 | 0.48 |
| Copper | 11.90 | 1.16 | 8.14 | 0.99 |
| Iron | 43.10 | 0.48 | 28.50 | 0.37 |
| Manganese | 11.60 | 0.77 | 12.40 | 0.96 |
| Nickel | 3.21 | 0.47 | 0.22 | 0.38 |
| Lead | <0.20 | <0.20 | ||
| Vanadium | 0.81 | 1.19 | 0.79 | 1.02 |
| Zinc | 26.80 | 1.12 | 19.2 | 1.14 |
| Selenium | <0.10 | <0.10 | ||
| Antimony | <0.05 | <0.05 | ||
| Mercury | <0.05 | <0.05 | ||
| Magnesium | 1136. | 1.92 | 1470. | 2.44 |
| Calcium | 788. | 2.97 | 1001. | 4.02 |
| Sodium | 289. | 2.12 | 421. | 1.22 |
| Phosphorus | 5166. | 1.02 | 5019. | 0.99 |
Table 3: Micro-macroelements (μg/g) in styles of saffron crocus of the Fields A and B.
Comparison of the styles and pollen showed pollen with a higher tendency than the styles to store micro-elements, including detrimental trace elements. A reverse trend was for macro-elements, particularly the sodium which in the stigmas showed a concentration ten times greater than in pollen.
Corms from field B: In the corm the concentrations of the metals were all lower than the allowable levels (Table 4). The values were less or equal than those found in pollen, except for the aluminum and the iron whose values were higher.
| Corm Field B | RSD %a | Soil Field B | RSD %a | |
|---|---|---|---|---|
| Aluminium | 36.60 | 1.23 | 14229. | 5.34 |
| Antimonium | <b0.05 | <0.05 | ||
| Arsenic | <0.50 | 3.50 | 2.29 | |
| Cadmium | <0.05 | 2.90 | 0.19 | |
| Cobalt | <0.20 | 3.10 | 1.23 | |
| Cromum | <0.20 | 8.20 | 4.95 | |
| Iron | 39.40 | 1.47 | 5002. | 4.89 |
| Manganese | 6.60 | 0.08 | 249.60 | 2.35 |
| Mercury | <0.05 | 0.10 | 1.37 | |
| Nichel | <0.50 | 7.90 | 1.02 | |
| Lead | <0.20 | 8.00 | 1.02 | |
| Cupper | 3.70 | 1.38 | 36.80 | 3.02 |
| Selenium | <0.20 | <0.20 | ||
| Tin | 0.70 | 0.44 | 1.10 | 1.47 |
| Vanadium | <0.50 | 11.40 | 2.01 | |
| Zinc | 16.40 | 1.08 | 37.70 | 2.36 |
| Magnesium | 958 | 4.13 | n.d.c | |
| Calcium | 467 | 2.89 | n.d.c | |
| Sodium | 20.00 | 1.38 | n.d.c | |
| Phosphorus | 3369 | 1.15 | n.d.c |
Table 4: Micro-macroelements (μg/g) in saffron corms and in the soil of the field B.
Soil from field B: The soil of the Field B was contamined by metals (Table 4). The values were very high for aluminium and iron and high enough for the heavy metals which are very toxic such as lead, chrome, cadmium and arsenic most of which with values more than ten times higher than those recommended for the pollen [5].
The variety of valuable fatty acids found in both pollenkitt and anther wall, and the presence of beneficial mineral elements with low sodium concentration deserve attention, as well as the tendency of pollen and anther to absorb alarming doses of cadmium and arsenic. This vulnerability to certain metals, not detected in the female parts of the flower or in the underground plant, makes the stamens as specific indicators of soil contaminations. In the cultivation fields, saffron anthers with pollen can be easily collected during collection of styles, so they are not exposed to contact with insects and any pollutant because farmers collect the flowers before they open. As regards the water content in the pollen it is reduced to a minimum of 7% within 8-10 h [27] after the dehiscence of the anther, and this dryness is recommended for the quality of the pollen [5]. These characters of quality are not guaranteed in pollen commonly used as a food supplement like bee pollen which is mixed with the bee saliva and stored at the entrance of the hive, so it is exposed to moisture and contamination before be collected by the beekeeper. In addition, a mixture of pollen from different species such as bee pollen can increase the risk of allergic reactions to consumers.
In conclusion, this study points out that pollen and anthers of saffron can be a beneficial source of nutrients to be used as dietary supplements when grown in soils not contaminated by toxic heavy metals. No alarm of metal contamination for the female flower part which gives the saffron spice, and the corms that are the means of saffron plant propagation.
We are obliged to thank for their assistance and technical support for analysis with relevant instruments, Dr. Luciana Di Giacinto of Research Centre for Olive Growing and Oil Industry, Città S. Angelo (PE), Italy, and Dr. Domenica Flammini and Valentina Ferrari of Regional Agency for the Protection of the Environment, Provincial District of L’Aquila, Caselle di Bazzano (AQ), Italy.