Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2017) Volume 7, Issue 2
Background: Fruits and fruit products are important part of food and contribute significantly to the food security of the society especially in terms of vitamins and micronutrients. Objectives: To investigate the nutrient composition and phytochemical contents of the lyophilized-edible parts of Chrysophyllum albidum fruit. Methods: The edible parts (fruit-pulp, fruit-skin and seed-shell pericarp) of C. albidum were separated, lyophilized and pulverized into powdered. The edible parts were evaluated for phytochemicals constituents, proximate, fibers, sugars, starch and mineral elements using standard methods. Results: The fruit-skin in comparison with the fruit-pulp and seed shell pericarp had the highest proximate (except for crude fat, crude protein, carbohydrate and moisture); fiber fractions (except lignin), starch, minerals (except for chloride and iron) and phytochemical (except for alkaloids, tannin and vitamin C) values. Fruit-pulp had the highest lignin (3.16 ± 0.07%), iron (7.66 ± 0.02 mg/100 g) and sugar (except arabinose) contents. Seed shell pericarp had the highest chloride (81.28 ± 0.56 mg/100 g), alkaloids (25.80 ± 0.51%), tannin (10.19 ± 0.12%) and vitamin C (0.12 ± 0.01%) contents. Results are significantly (p<0.05) different between samples for sugar, starch, fat, calcium, magnesium, potassium, chloride, alkaloid, tannin and flavonoid contents. The Gas chromatography/Mass spectrometry analysis of n-hexane extract of the edible parts showed the presence of diverse phytochemicals which are mainly of antioxidants and fatty acid origins. Conclusion: The study established that the fruit-skin contained more essential nutrients composition than other samples investigated and with moderate bioactive compounds of diverse purposes.
Keywords: Nutrients; Chrysophyllum albidum ; Proximate; Minerals; Gas chromatography; Mass spectrometry
Fruits have been shown to be one of the best sources of dietary fiber with lots of vitamins especially C, E and A as well as minerals. Frequent consumption of fruits and vegetables has been associated with a lowered risk of diabetes, hypertension, coronary heart disease, cancer, and stroke [1,2]. Fruits give carbohydrates in the form of soluble sugars, cellulose and starch [3] and serve as source of nutrient, appetizer and supplement for food in a world faced with problem of food scarcity.
Chrysophyllum albidum (Linn), also known as African star apple, belongs to the family Sapotaceae , is primarily a forest tree species (Figure 1a) with its natural occurrences in diverse ecozones in Uganda, Nigeria and Niger Republic [4]. The fruit (Figure 1b) is seasonal (December-April) and has immense economic potential, especially following the report that jams obtained from the fruit-pulp could compete with raspberry jams and jellies [5] while the oil from the seed has been used for diverse purposes [6]. In Nigeria, C. albidum is known as ‘‘agbalumo’’ in South Western Nigeria and “udara” in South Eastern Nigeria. Its rich sources of natural antioxidants have been established to promote health by acting against oxidative stress related diseases such as diabetics, cancer and coronary heart diseases [7].
Figure 1: Chrysophyllum albidum tree (A) and fruits (B) [17].
The fruit-pulp has been reported to contain significant amount of ascorbic acid [8], vitamins, iron and food flavors [9], fat [10,11], carbohydrate and mineral elements [8,10-13]. The fruit-peel has been shown to be a rich source of fiber and mineral [10,14] while the seed shell pericarp has been reported to be a good source of carbohydrate and minerals [15]. The fruits are not only consumed fresh but also used to produce stewed fruit, marmalade, syrup and several types of soft drinks [16].
Proximate and nutrient analysis of edible fruits and vegetables plays a crucial role in assessing their nutritional significance [17,18]. Freeze-drying method is a drying technique considered appropriate for the preservation of nutritional values of the dehydrated products due to the absence of liquid water and the low temperatures required in the process [19].
In spite of wide application of C. albidum plant and its great potential as good sources of fiber and carbohydrate, information on the fiber fractions and sugar contents of its edible parts seems to be scanty in the available literature has not been fully investigated. This study was designed to evaluate the nutritional and phytochemical contents of the edible parts (seed shell pericarp, fruit pulp and fruit-skin) of C. albidum fruit. The results of this study may provide useful information on its nutritional potential and contribution to nutrient intake of the nation. It may also create public awareness of its utilization when in season.
Plant materials
The fresh fruits of C. albidum were purchased in Moniya market, Akinyele Local Government Area of Oyo State, South-Western Nigeria. The fruit was identified and authenticated in the herbarium unit of Botany Department, University of Ibadan, Oyo State, Nigeria where a voucher specimen was deposited with the voucher registration No. UIH/20I6/22502.
Preparation of plant materials
The fresh ripe fruit of C. albidum was separated, washed, weighed and its fruit-pulp, fruit-skin and seed-shell pericarp (samples) removed and cut into small pieces. The samples were lyophilized for 54 h using Lyophilizer, Millorock Bench-Top Freeze Dryer, and Germany. Lyophilized samples were stored at -20ºC until further use.
Extraction of plant materials
The lyophilized samples (fruit-pulp, fruit-skin and seed-shell pericarp) of C. albidum fruit were pulverized into powder using Thomas-Wiley laboratory mill (model 4) before being extracted. A portion of one hundred grams (100 g) each of the pulverized sample was suspended in 800 ml of n-hexane for 48 hrs using cold maceration methods with intermittent shaking [20]. The crude extract was filtered first with cotton wool followed by Whatman No. 1 filter paper (1 mm mesh size), concentrated in a rotary evaporator at 40?C and dried to completion in water bath at 35ºC. The crude extract was kept in sealed containers and refrigerated at 2-4ºC for GC-MS analysis.
Qualitative phytochemical screening
Preliminary qualitative phytochemical screening of the samples was determined using the methods [21-23].
Determination of Tannin
Tannin content of the samples was determined based on the modified vanillin-HCI menthanol method as described by Noha et al. [24]. The principle is that tannins are usually extracted using aqueous organic solvents, mainly methanol and acetone. Vanillin reacts with proantocyandins and leucoanthocyanidins or catechins in the presence of HCI giving rise to a bright red color.
Procedure:
Serial dilution of 1 mg/ml solution A (0.05, 0.1, 0.3, 0.4, 0.5) ml was mixed with 3 ml of 1% vanillin prepared in methanol and 1.5 ml of HCI and made up to a total volume of 5 ml using distilled water. About 0.2 g of the ground sample was placed in a small conical flask. Then 10 ml of 1% concentrated HCI in Methanol was added. The flask was capped, shaken continuously for 20 min and the content was further centrifuged at 2500 rpm for 5 min. About 1.0 ml of the supernatant was pipetted into a test tube containing 5 ml of Vanillin–HCI reagent (solution A). Absorbance at 450 nm was read on spectrophotometer after 20 min of incubation at 30ºC. A standard curve was prepared expressing the result as tannic acid equivalent as follow:
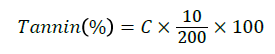
Where C=Concentration corresponding to the optical density; 10=Volume of the extract (ml)=Sample weight (mg).
Determination of Saponin
Saponin content determination was carried out according to the procedure described by Okwu and Josiah [25]. 5 g of the sample was dispersed in 50 ml of 20% v/v ethanol prepared in distilled water. The suspension was heated over hot water bath for 4 h with continuous stirring at 55ºC. The mixture was filtered and the residue re-extracted with another 50 ml of 20% ethanol. The combined extracts were reduced to 20 ml in a hot water bath. The concentrated solution obtained was shaken vigorously with 10 ml of diethyl ether in a 250 ml separating funnel; the aqueous layer was collected while the ether layer was discarded. The purification process was repeated twice. Twenty milliliters but-1-ol was added to the filtrate and washed twice with 10 ml of 5% w/v aqueous sodium chlorine. The mixture heated to evaporation on hot water bath and later oven dried at 40ºC to a constant weight. The percentage saponins content of the sample was calculated using this formula:
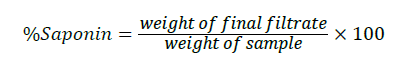
Determination of alkaloids
This was done by the alkaline precipitation gravimetric method described by Harborne [23]. One gram of the sample was dispersed into 10 ml of 10% acetic acid solution in ethanol to form a ratio of 1:10 (10%). The mixture was shaken and then allowed to stand for 4 h before it was filtered. The filtrate was concentrated to one quarter of its original volume on hot plate and treated with drop wise addition of conc. aqueous NH4OH until the alkaloid was precipitated. A pre-weighed filter paper was used to receive the precipitate, washed with 1% ammonia solution, dried in an oven at 60ºC for 30 min, transferred into desiccators to cool; rewashed and dried until a constant weight was obtained. The constant weight was recorded and alkaloid content was determined by weight difference of the filter paper and expressed as a percentage of the sample analyzed.
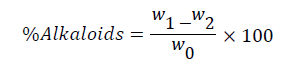
Where WO=Sample weight in g; W1=Weight of filter paper +precipitate.
W2=weight of filter paper only.
Determination of flavonoids
This was determined according to the method of Harborne [23]. 5 g of the sample was boiled in 50 ml of 2 M HCl solution for 30 min under reflux. It was allowed to cool and then filtered. About 2 ml of the filterate was treated with 2 ml ethyl acetate starting with a drop. The flavonoid precipitated was recovered by filtration using weighed filter paper. The resulting weight difference gave the weight of flavonoid in the sample.
Determination of vitamin C
Vitamin C content was determined by UV-spectrophotometry as described by Rahman et al. [26]. One gram (1g) each of sample was weighed into test tubes. 1 mm ascorbic acid stock was pipetted into a separate test tube as a standard. 1 mm Trichloreacetic Acid (TCA) solution was placed in another test tube to serve as blank. Ten millilitre TCA solutions were added to the sample tubes. 1 mm Dinitrophenyl Hydrazine-Thiourea-Copper Sulphate (DTCS) reagent was added to all the tubes and caped. The tubes were incubated in a water bath at 37ºC for 3 hrs. They was removed from the water bath and chilled for 10 min in an ice bath while shaking slowly and 2 ml of cold 12 M H2SO4 was added to all the test tubes. The spectrophotometer was adjusted with the blank to read zero absorbance at 520 nm. The absorbance of standard and test samples was also read. The result was calculated as follows:
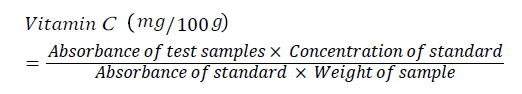
Proximate Composition
Moisture, ash and crude fibre contents of the samples were determined by the AOAC [27] methods. The micro-Kjeldahl method was used for the determination of crude protein (N × 6.25) using the methods of AOAC [27]. Crude lipid contents were determined by the continuous soxhlet lipid extraction method was determined by the method of AOAC [27]; the carbohydrate content was estimated by difference [28].
Characterization of carbohydrate content
The characterization of carbohydrate content of the samples was done with HPLC using the method described by Prapasri and Kunchit [29].
Determination of mineral content
Sodium (Na) and potassium were determined by the flame emission photometer using the AOAC [27], Phosphorus (P) was determined by vanadomolybdate colorimetric method, Calcium (Ca), Magnesium (Mg), Iron (Fe), zinc (Zn), manganese (Mn), chromium (Cr) and copper (Cu) were determined using Atomic Absorption Spectrophotometer (AAS) [30] and Chloride by Mohr’s Method [31]. All values were expressed in mg/100 g.
Starch and fiber fractions determination
The total starch was determined by the method of McCleary et al. [32]. The Neutral Detergent Fibre (NDF), Acid Detergent Fraction (ADF), hemicellulose, cellulose and lignin were determined using fiber analyzer [33]. Pectin was determined by nitric acid extraction method and percentage content was calculated by non-enzymatic gravimetric method [34].
Gas chromatography-mass spectrometry (GC-MS) analysis
The GC-MS analysis of n-hexane extract of the samples were carried out using a Perkin-Elmer GC Clarus 500 system and Gas chromatograph interfaced to a Mass spectrometer (GC-MS) equipped with an Elite-I, fused silica capillary column (30 × 0.25 mm 1 D × 1 μMdf, composed of 100% Dimethyl poly siloxane). For GC-MS detection, an electron ionization system with ionization energy of 70 eV was used. Helium gas (99.999%) was used as the carrier gas at constant flow rate 1ml/min and an injection volume of 2 μl was employed (Split ratio of 10:1) injector temperature -250ºC; ion-source temperature 280°C. The oven temperature was programmed from 110ºC (Isothermal for 2 min.) with an increase of 10ºC/min to 200ºC then 5ºC/min. to 280ºC/min, ending with a 9 min. isothermal at 280ºC. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 40 to 550 Da. Total GC running time was 36 minutes.
Identification of components
Interpretation on mass spectrum GC-MS was conducted using the database of National Institute Standard and Technology (NIST) having more than 62,000 patterns. The spectral of the unknown component were compared with the spectral of the known components stored in the NIST library. The possible names, molecular weights and structures of the components of the test materials were thus ascertained [35].
Data were expressed as mean ± SEM for triplicate determinations (n=3). Comparisons of the data were performed by analysis of variance (ANOVA) followed by Least Significant Difference (LSD) post hoc test at 5% (p<0.05) probability.
Qualitative phytochemical
Preliminary phytochemical investigation (Table 1) of the crude edible parts of Chrysophyllum albidum fruit revealed the presence of alkaloids, tannins, saponins, flavonoids and terpenoids in all the edible parts of C. albidum. Cardiac glycosides was found to be absent in the fruit-skin while phlobatannin was also found to be absent in both seed shell pericarp and fruit-skin of C. albidum.
| Parameters | Seed shell pericarp | Fruit pulp | Fruit skin |
|---|---|---|---|
| Alkaloid | + + + | + + + | + + + |
| Tannin | + + | + + | + + |
| Saponin | + | + | + |
| Flavonoid | + + + | + + + | + + + |
| Terpenoid | + + + | + + + | + + + |
| Phlobatannin | - | + | - |
| Cardiac glycoside | + | + | - |
| (+): Presence (trace amount), (++): Presence (moderately concentrated), (+++): Presence (highly concentrated), (- ): Absent | |||
Table 1: Phytochemical screening of the edible parts of freeze dried Chrysophyllum albidum fruit.
Quantitative phytochemicals
The quantitative phytochemical values of C. albidum edible parts are shown in Table 2. Seed shell pericarp had the highest contents of Alkaloid, Tannins and vitamin C. while the Fruit skin had the highest content of Saponin and Flavonoid. Data obtained between samples with the exception of Vitamin C are significantly (P<0.05) different.
| Parameters (%) | Seed shell pericarp | Fruit pulp | Fruit skin |
|---|---|---|---|
| Alkaloid | 25.80a ± 0.51 | 14.60b ± 0.16 | 12.22c ± 0.10 |
| Tannin | 10.19a ± 0.12 | 7.49c ± 0.12 | 8.18b ± 0.07 |
| Saponin | 0.09b ± 0.01 | 0.07b ± 0.02 | 0.41a ± 0.02 |
| Flavonoid | 15.11b ± 0.07 | 11.24c ± 0.15 | 17.23a ± 0.09 |
| Vitamin C (mg/100g) | 0.12a ± 0.01 | 0.10a,b ± 0.01 | 0.09b ± 0.01 |
| Results are the mean ± SE value of triplicate determinations. Means followed by different alphabet within the row are significantly (P<0.05) different while those with the same alphabet within the row are not significantly (P>0.05) different. | |||
Table 2: Quantitative phytochemical constituents of the freeze dried edible parts of Chrysophyllum albidum fruit.
Proximate composition and starch content
Data on proximate composition and starch content are presented in Table 3. The seed shell pericarp had the highest crude protein, crude fat and energy contents; fruit-pulp had the highest moisture and carbohydrate contents while fruit skin had the highest ash, crude fibre, total dry weight and starch contents. The values are significantly different (p<0.05) across samples (with the exception of ash, crude protein and carbohydrate).
| Parameters (%) | Seed shell pericarp | Fruit pulp | Fruit skin |
|---|---|---|---|
| Moisture | 5.63b ± 0.24 | 7.76a ± 0.15 | 4.80c ± 0.15 |
| Ash | 2.36b ± 0.07 | 2.29b ± 0.04 | 3.27a ± 0.13 |
| Crude Protein | 8.66a ± 0.19 | 7.31b ± 0.46 | 6.91b ± 0.48 |
| Fat | 8.07a ± 0.13 | 6.97b ± 0.51 | 4.68c ± 0.23 |
| Crude Fiber | 10.06b ± 0.14 | 9.21c ± 0.19 | 15.57a ± 0.25 |
| Carbohydrate | 65.22a ± 0.61 | 66.45a ± 0.65 | 64.70a ± 0.21 |
| TDW | 94.37a ± 0.22 | 92.24b ± 0.15 | 95.20a ± 0.31 |
| Gross Energy (kj/100 g) | 1537.91a ± 7.23 | 1494.74b ± 5.52 | 1373.31c ± 5.10 |
| Starch | 20.86b ± 0.22 | 13.13c ± 0.12 | 29.15a ± 0.11 |
| Results are the mean ± SE value of triplicate determinations. Means followed by different alphabet within the row are significantly (P<0.05) different while those with the same alphabet within the row are not significantly (P>0.05) different. TDW: Total Dry Weight. | |||
Table 3: Proximate Composition of the freeze dried edible parts of Chrysophyllum albidum fruit.
Fiber, sugar and mineral contents: The Fiber fractions are presented in Figure 2. The fruit skin had the highest contents of Acid Detergent Fraction (ADF), Neutral Detergent Fiber (NDF), cellulose, hemicelluloses and pectin while the fruit pulp had the highest contents of lignin. Values are significantly (p<0.05) different across samples except pectin where P>0.05. The sugar contents of C. albidum fruit showed that the seed shell pericarp had the highest contents of the sugar studied except arabinose while the fruit skin had the highest content of arabinose and lowest contents of other sugar studied (Table 4). The mineral contents C. albidum fruit (Tables 5 and 6) showed that the fruit skin had the highest contents of the mineral elements studied except chloride and iron while the highest chloride and iron contents were found in the seed shell pericarp and fruit pulp respectively. Values are significantly (P<0.05) different across samples except for copper.
| Sample/Parameters (mg/100 g dry wt) | Glucose | Fructose | Arabinose | Sucrose |
|---|---|---|---|---|
| Seed Shell Pericarp | 8133.61b ± 20.50 |
14146.98b ± 32.55 | 3523.06b ± 14.84 | 1095.66b ± 14.05 |
| Fruit Pulp | 13937.01a ± 20.40 | 16574.10a ± 35.77 | 3294.55c ± 12.10 | 8236.61a ± 21.89 |
| Fruit Skin | 7764.02c ± 11.45 |
10880.41c ± 7.33 |
4854.79a ± 12.40 | 67.27c ± 0.08 |
| Results are the mean ± SE value of triplicate determinations. Means followed by different alphabet within the row are significantly (P<0.05) different while those with the same alphabet within the row are not significantly (P>0.05) different. | ||||
Table 4: Sugar contents of the edible parts of Chrysophyllum albidum fruit.
| Parameters (mg/100 g dry wt) | Seed Shell Pericarp | Fruit Pulp | Fruit Skin |
|---|---|---|---|
| Na | 28.26c ± 0.02 | 31.03b ± 0.09 | 34.00a ± 0.71 |
| K | 532.08b ± 33.71 | 268.00c ± 0.55 | 585.75a ± 0.78 |
| Mg | 122.41c ± 0.20 | 135.00b ± 1.80 | 144.25a ± 1.02 |
| Ca | 212.50b ± 0.51 | 100.00c ± 5.51 | 258.25a ± 6.86 |
| Cl | 81.28a ± 0.56 | 24.74c ± 0.43 | 70.68b ± 0.40 |
| P | 12.04b ± 0.00 | 10.03b ± 0.00 | 30.22a ± 0.01 |
| Results are the mean ± SE value of triplicate determinations. Means followed by different alphabet within the row are significantly (P<0.05) different while those with the same alphabet within the row are not significantly (P>0.05) different. | |||
Table 5: Macro elements content of the edible parts of freeze dried Chrysophyllum albidum fruit.
| Parameters (mg/100 g dry wt) | Seed Shell Pericarp | Fruit Pulp | Fruit Skin |
|---|---|---|---|
| Fe | 3.48b ± 0.17 | 7.66a ± 0.02 | 3.37b ± 0.15 |
| Zn | 0.12b ± 0.02 | 0.57a ± 0.04 | ND |
| Mn | 0.23c ± 0.03 | 0.45b ± 0.02 | 2.25a ± 0.06 |
| Cr | ND | 0.25 ± 0.02 | ND |
| Cu | 0.36a ± 0.02 | 0.44a ± 0.04 | 0.55a ± 0.05 |
| Results are the mean ± SE value of triplicate determinations. Means followed by different alphabet within the row are significantly (P<0.05) different while those with the same alphabet within the row are not significantly (P>0.05) different. ND: Not Detected. | |||
Table 6: Micro elements content of the edible parts of freeze dried Chrysophyllum albidum fruit.
Results are expressed as the mean ± SE value of triplicate determinations. All values are significantly different (P<0.05) with the exception of pectin and hemicelluloses (between seed shell pericarp and fruit pulp).
GC-MS analysis
The GC-MS identified compounds and their reported bioactivity in the n-hexane extract of the edible parts of C. albidum with percentage of purity ≥70% is presented in Table 7. The identified compounds are of fatty acids, fatty acid derivatives and aromatic origin. The studies showed that they are rich in phytochemicals of diverse nutritional and biological significance. Result of n-hexane extract of C. albidum seed shell pericarp showed that it contained 6 compounds, 1 of these compounds is known to exhibit both antioxidant and hypocholesterolemic activities; and 1 compound is known to exhibit hypolipidemic activity while 2 compounds of unknown bioactivity were also detected.
| Sample type | Compound name | Retention time | Compound Nature | Activity* |
|---|---|---|---|---|
| Seed shell pericarp | Pentadecanoic acid | 13.793 | Fatty acid | Antimicrobial |
| n-Hexadecanoic acid | 15.727 | Fatty acid | Antioxidant Hypocholesterolemic | |
| Trans-13-Octadecenoic acid | 16.934 | Trans Monoenoic Fatty acid | # | |
| 6-Octadecenoic acid | 17.003 | Petroselinic acid | # | |
| Cis-Vaccenic acid | 17.804 | Monoenoic Fatty acid | Hypolipidemic | |
| Octadecanoic acid | 18.044 | Fatty acid | Antifungal, antitumor | |
| Fruit pulp | Naphthalene | 6.217 | Bicyclic Aromatic Hydrocarbon | Anti-inflammatory, Antimicrobial |
| Tetradecane | 8.860 | Alkane | Antifungal, Antibacterial | |
| Undecanoic acid, 10-methyl-methyl ester | 10.434 | Fatty acid ester | # | |
| 9-Ecosene | 11.246 | Alkene | # | |
| Hexadecane | 11.327 | Alkane | # | |
| Benzene(1-propyloctyl)- | 11.939 | Aromatic compound | # | |
| Methyl tetradecanoate | 12.757 | Myristic acid ester | Antioxidant, Hypocholesterolemic | |
| Benzene(1-pentylheptyl) | 12.866 | Aromatic compound | # | |
| Benzene(1-butyloctyl) | 12.923 | Aromatic compound | # | |
| Benzene(1-propylnonyl) | 13.060 | Aromatic compound | # | |
| Benzene(1-ethyldecyl) | 13.306 | Aromatic compound | # | |
| Trifluoroacetoxy hexadecane | 13.490 | Fluoro compound | Antifungal | |
| Octadecane | 13.558 | Alkane | # | |
| Hexadecanoic acid, methyl ester | 14.863 | Palmitic acid methyl ester | Antioxidant, Hypocholesterolemic | |
| Hexadecanoic acid ethyl ester | 15.521 | Palmitic acid ethyl ester | Antioxidant, Hypocholesterolemic | |
| Eicosane | 15.572 | Alkane | Antifungal, Antitumor | |
| 9,12-Octadecadienoic acid-methyl ester | 16.516 | Linoleic acid methyl ester | Anti-inflammatory, Hypocholesterolemic | |
| 11-Octadecenoic acid, methyl ester | 16.562 | Vaccenic acid methyl ester | Hypolipidemic | |
| Octadecanoic acid, methyl ester | 16.774 | Stearic acid methyl ester | Antifungal, Antimicrobial | |
| Cyclohexene,4-(4-ethylcyclohexyl) | 17.123 | Cyclo Hydrocarbon | # | |
| Octacosyl trifluoroaacetate | 17.369 | Fluoro Compound | Antimicrobial | |
| Docosane | 17.421 | Alkane | Antibacterial | |
| Sulfurous acid, Octadecyl-2-propyl ester | 19.103 | Sulfurous compound | Antimicrobial | |
| Fruit skin | Hexadecane | 11.332 | Alkane | # |
| Methyltetradecanoate | 12.757 | Myristic acid ester | Antioxidant, Hypocholestero-lemic | |
| 1-octadecene | 13.489 | Alkene | # | |
| Octadecane | 13.558 | Alkane | # | |
| Hexadecanoic acid methyl ester | 14.863 | Palmitic acid methyl ester | Antioxidant, Hypocholestero-lemic | |
| 3-Eicosene | 15.521 | Alkene | # | |
| Eicosane | 15.578 | Alkane | Antifungal, antitumo | |
| 9,12-octadecadienenoic acid, methyl ester | 16.522 | Linoleic acid methyl ester | Anti-inflammatory, Hypocholesterolemic | |
| 9-Octadecenoic acid, methyl ester | 16.568 | Oleic acid methyl ester | Antioxidant, Hypocholestero-lemic | |
| Octadecanoic acid, methyl ester | 16.780 | Stearic acid methyl ester | Antioxidant, Hypocholestero-lemic |
Table 7: Phytochemicals of n-hexane extract of freeze dried edible parts of Chrysophyllum albidum Fruit.
The n-hexane extract of C. albidum fruit-pulp contained 23 compounds, 3 of these compounds are known to exhibit both antioxidant and hypocholesterolemic activities; 1 compound exhibits both anti-inflammatory and hypocholesterolemic activities; 1 compound exhibits hypolipidemic activity; and 1 compound exhibits only anti-inflammatory activity while 10 compounds of unknown bioactivity were also detected. The n-hexane extract of C. albidum fruit-skin contained 10 compounds, 3 of these compounds are known to exhibit both antioxidant and hypocholesterolemic activities, 1 compound exhibits anti-inflammatory, antioxidant and hypocholesterolemic activities, and 1 compound exhibits both antiinflammatory and hypocholesterolemic activities; while 4 compounds of unknown bioactivity were also detected.
ADF: Acid Detergent Fraction; NDF: Neutral Detergent Fiber.
The health promoting properties of plant-based foods have largely been attributed to their wide range of phytochemicals [36-38]. The presence of flavonoids, alkaloids, terpenoid, tannins and saponin in the edible parts of C. albidum fruit agreed with the reports of Egharevba et al. [39] who reported the presence of phenols, glycosides, terpenoids in the leaf; flavonoids, saponins, steroids and alkaloids in the leaf and seed of Chrysophyllum albidum methanol extract. This work is also in line with the findings of Imaga and Urua [40] who showed that ethanol and aqueous extracts of Chrysophyllum albidum fruit contain tannins, phenols, flavonoids, cardiac glycosides, terpenoids, reducing sugar and phlobatannins while saponins, steroids and alkaloid were reported to be present only in the aqueous extract and anthraquinone found to be absent in both extracts. These metabolites are of great importance in phytomedicines development [21].
Flavonoids and Tannins in fruits are important sources of natural antioxidants that are preferred over synthetic ones as they are less toxic [41]. Flavonoids scavenge free radicals produced by Reactive Oxygen Species (ROS) thereby preventing diseases caused by oxidative stress. Alkaloids have anti-inflammatory property [42] while saponins exhibit hypocholesterolemic property through the formation of insoluble complexes with cholesterol and consequently slowing down its absorption [43]. The freeze dried edible parts of C. albidum upon quantification by colorimetric methods were found to be rich in alkaloids, tannins and flavonoids with moderate amount of saponin. This indicates that the edible parts of C. albidum fruit might possess antioxidant, anti-inflammatory and hypocholesterolemic properties.
There is a growing awareness in correlating the phytochemical components and their biological activities [44]. The GC-MS analysis of n-hexane extract of edible parts of C. albidum fruit showed the presence of various bioactive compounds. In terms of biological activity six of the compounds possess both antioxidant and hypocholesterolemic properties. These compounds are n-hexadecanoic acid; methyl tetradecanoate; hexadecanoic acid, methyl ester; hexadecanoic acid, ethyl ester; 9-octadecenoic acid, methyl ester and octadecanoic acid, methyl ester. Three compounds (cis-vaccenic (11– octadecenoic) acid; 11–octadecenoic acid, methyl ester and 9, 12- Octadecadienoic acid, methyl ester) possess only hypocholesterolemic property.
Daily diet has an important role in human health and healthy diet means getting enough of the essential nutrients needed daily by the body. Proximate and nutrient analysis of edible fruit and vegetables play a crucial role in assessing their nutritional significance. The obtained moisture contents are lower than the values reported for this fruit seed shell pericarp by Ewansiha et al. [15]; pulp by Ukana et al. [14]; Bello and Henry [16] and skin (peel) by Bello and Henry [16]. The observed low moisture content could be due to the shielding effect of the freeze drying method over air drying method on the samples against rehydration by relative humidity in air [45]. This result is in line with the findings of Abiodun et al. [46] who reported that freeze drying method is the most appropriate method to reduce moisture content of Ocimum gratissimum and O. basillicum . The moisture content of any food material is a measure of the life span of the food. It indicates how long a food material can be stored without becoming mouldy [47]. Hook et al. [48] reported that the moisture level of a food influence the texture and the more ordered the endosperm structure, the lower the rate of moisture content. This indicates that freeze dried samples could be stored for long period without becoming mouldy.
The obtained ash contents are in close range with the findings of Amusa et al. [6]; Ureigho [13]; Christopher and Dosunmu [11]; Ewansiha et al. [15] and Ukana et al. [14]. Ash content indicates the amount of inorganic matter and oxides present in the sample. It is the determinant factor of the mineral constituents in the sample [17]. Proteins are class of nitrogenous compound that consist of large molecule composed of one or more long chain of amino acid. The obtained protein values are higher compared to the values reported for this fruit by Amusa et al. [6]; Ewansiha et al. [15] and Ukana et al. [14].
However, the observed crude fat values agreed with the reports of Christopher and Dosunmu [11]; Ewansiha et al. [15] and Ukana et al. [14] for this fruit. Fat provide an excellent source of energy, enhance transport of fat soluble vitamins, insulate and protect internal tissues and contribute to vital cell process. However, it is strongly believed that excess of saturated fatty acids are responsible for a tendency to coronary thrombosis and aortic atheroma in men while high level of poly unsaturated fatty acids are important in lowering blood cholesterol level.
The obtained crude fiber content are higher than the values reported for this fruit by Amusa et al. [6]; Ewansiha et al. [15]; Bello and Henry [16]. These variations could be due to different methods of drying effects. This result is in line with the findings of Adejumo [49] who reported significant effects of different drying methods on crude fiber content of tomato powder and Oni et al. [45] who showed an increasing effect of drying methods on the crude fiber contents of different vegetables in the order of freeze drying>Air drying>Sun drying>Oven drying. Fiber decreases the absorption of cholesterol from the gut, delays the digestion and conversion of starch to simple sugars, and also functions in the protection against cardiovascular disease, colorectal cancer and obesity [50]. The fiber-like substances particularly the gums and pectin when ingested with a diet have been reported to reduce postprandial blood glucose levels [51,52]. Feeding of xanthan gum to diabetic subjects have been shown to lower fasting and postload serum glucose levels and total plasma cholesterol [53]. Daily soluble fiber intake of 5–10 g from a variety of sources has been found to reduce serum cholesterol by 5–10% [51]. This implies that the fruit-skin with the highest fiber fractions could be effectively utilized in the management of diabetes mellitus, colorectal cancers and weight reduction in the obese individuals. However, the fruit-skin will take longer time to chew because of its fiber-rich contents.
The obtained carbohydrate contents are consistent with the reports of Christopher and Dosunmu [11]; Ewansiha et al. [15] and Ukana et al. [14] for this fruit. Polysaccharides such as starch have low glycemic index and thus effective in the control of postprandial blood glucose levels. The observed high starch content in the C. albidum fruit skin suggests that the fruit skin could reduce glycemic response when ingested with carbohydrate diet. However, the low caloric value of the fruit skin may be a disadvantage for its suitability for consumption as a snack by the athletes.
There is evidence for a direct, independent link between sugar exposure and diabetes and increased access to sugar may explain part of the association between urbanisation and increased risk of type 2 diabetes [54]. The high content of arabinose and low content of fructose in the fruit skin have beneficial effects. Diet high in fructose has been reported to promote energy imbalance, obesity [55]; glucose intolerance, insulin resistance, hyperlipidaemia [56] and total cholesterol/HDL-cholesterol ratio which are known to be high-risk factors for development of cardiovascular diseases [57]. Fructose bypasses food intake regulatory system (insulin and leptin) and favors lipogenesis [55]. This implies that the high fructose content of the fruit pulp may be a disadvantage for its suitability for consumption as a snack by the obese.
Studies using animal model and clinical trials have shown that L-arabinose supplemented diet could effectively reduce postprandial blood glucose level by uncompetitive inhibition of intestinal sucrose [58,59]. This implies that the fruit skin might possess ability to suppress glycaemic response when taken with carbohydrate diet.
Minerals are critical in the regulation of a number of cell membrane, permeability, muscles contraction, heart function, blood clothing, protein and red blood synthesis [60,61]. These essential minerals are important components of the daily diet. The high contents of potassium, magnesium, calcium, phosphorus, sodium, manganese and copper in the fruit skin, chloride content in the seed shell pericarp and iron content in the fruit pulp is an indication that the edible parts of C. albidum fruit can supply some essential minerals needed for healthy life.
Calcium, magnesium, manganese and copper have been shown to have stimulatory effect on the pancreas leading to enhanced insulin secretion and ß-cell proliferation, thus contributing to improved glucose disposal [62]. This suggests that the fruit skin with the highest contents of calcium, magnesium, manganese and copper could contribute to the control of hyperglycaemic cases through the enhancement of insulin secretion.
As a result of the phytochemicals and essential nutrients in the form of soluble and insoluble fibers, starch, arabinose and mineral elements found in the fruit skin of C. albidum in comparison with the seed shell pericarp and fruit pulp, it can be deduced that the fruit skin of C. albidum has a great potential in contributing to the healthy growth and as supplementation in food industries.
Further studies are required to elucidate the antioxidant, hypocholesterolemic, hypolipidemic and hypoglycemic potentials of C. albidum edible parts.
This research was supported in part by grant provided by the Education Trust Fund (ETF) of the Federal Government of Nigeria. The authors declare that there are no conflicts of interest.