Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Thesis - (2015) Volume 5, Issue 4
Pumpkin (Curcurbita maxima) is a popular vegetable in our country. This plant is locally known as “Mistikumra”. The seeds of pumpkin are rich in oil and nutrients. The nutritional compositions of pumpkin seeds were determined by standard method. The proximate compositions of the powdered seed were moisture 4.06%, ash 3.80%, crude fibre 2.91%, total lipid 36.70%, total protein 34.56%, total soluble protein 18.10%, sugar 1.08%, and starch 2.15%. The minerals composition of the seed were nitrogen 5.53%, phosphorus 0.71%, sodium 4.80 Cmol/kg, potassium 20.00 Cmol/kg, Calcium 4.40 Cmol/kg, Magnesium 348.7 ppm, iron 290.0 ppm, coper 70 ppm, zinc 39.9 ppm, and manganese 17.9 ppm. It is used as a potentially attractive source of lipid, protein and crude fibre. The oil was extracted from seed of Cucurbita maxima by solvent extraction process using petroleum ether (40°-60°C). The oil content was found to be 12%. The specific gravity of the oil was estimated to be 0.9412 at 31°C. GLC analysis of oil indicated that it contained highest amount of oleic acid 40.58%, while stearic acid, palmatic acid and linoleic acid contents were found to be 27.06%, 17.39% and 14.97% respectively. In addition, the iodine value, saponification value, saponification equivalent, acid value and percentage of free fatty acid of seed oil were determined by standard method and found to be 114.33, 193.73, 289.58, 0.516 and 0.2646% respectively. High degree of unsaturation makes it suitable for using as valuable drying agent, and lower free fatty acid content indicates suitability of the oil for probably edible purpose. Cururbita maxima seed oil is a rich source of linoleic acid, which is useful in human body.
Keywords: Curcurbita maxima, Nutrition, Lipid, Mistikumra, GLC
Good health is achieved by eating the proper kinds of food and vegetable. Well-balanced human foods and vegetables should contain adequate amount of nutrients, the shortage of which leads to malnutrition, which is common in West Africa [1]. Malnutrition is a major health problem in Cameroon, despite government’s efforts to promote food production. Protein-energy malnutrition in infant and children is one of the most common nutritional problems in Cameroon today [2].
A large number of populations in Bangladesh have been suffering from malnutrition. There are many kinds of plants available in Bangladesh, which are rich in nutrient. For the ignorance of people, they do not know the nutritive value of different kinds of vegetables.
Curcurbita maxima seed has an important role as a source of lipids, proteins, carbohydrates and other nutrients in human diet which are necessary for maintaining proper health [3]. Curcurbita maxima seed may be very important economic source of proteins, minerals, calories and of vitamins, which are essential for human nutrition. Although the seed of pumpkin is rich in oil and protein and the detailed study on their composition and properties of their oil is limited. The objective of this study was, therefore, the investigation of the nutritional composition of pumpkin seed.
They are one of the three major ‘Food groups’ needed for human body, the other two being proteins and carbohydrates. Fats and oils are widely distributed in food and are of great nutritional value. They provide concentrated reserve of energy in animal body for maintaining optimum body temperature. One gram of metabolized fat or oil yields 9 Kcal .While the corresponding value of carbohydrate and protein is 4 Kcal. and 5.5 Kcal respectively [4]. At present, human race uses an estimated 40 million tons of fats and oils in a year which reflects both their nutritional and industrial importance; and it is increasing daily in a very large magnitude. Drying oils are mainly used for making paints and varnishes; and also used for making linoleum and oil cloth. Some common drying oils are tung oil, linseed oil, perilla oil etc., used as cooking oil. Coconut oil, sesame oil, almond oil is also used as hair oil. Non-drying oils are used as materials of food, cooking oil and condiment. Some common non-drying oils are almond oil, palm oil, olive oil, lard, beef, mutton tallow oil etc.
The proximate compositions evaluation from the seed of C. maxima reveals protein 33.48%, carbohydrate 28.68%, lipid 30.66%, fiber 3.07%, ash 3.98%, and available energy 524.58 Kcal [5]. Isolation of oil has been reported from seed of several plants in order to make their utilization properly. The extracts of Curcurbita maxima seed show antidiabetic, anti-hyperlipidemic lowering both total cholesterol and triglyceride and at the same time increase HDL-cholesterol in STZinduced diabetic rats [6].
There has been evidence in recent years that the presence of large amount of saturated fat in the diet may lead to an increase in the level of cholesterol in blood, while the high oil content of the diet tends to diminish cholesterol level in blood.
The above-mentioned investigations and reports make us much interested to carry out research work on fats and oils from pumpkin seed.
Collection and processing
Curcurbita maxima, commonly known as “Mistikumra” in our country, was collected in 2007 from experimental plot located at Naoudapara, in the district of Rajshahi, Bangladesh. It was cultivated in homogeneous condition. The seeds were dissected from the vegetable and foreign materials were removed. After then, the seeds were dried in the sunlight for four consecutive days and crushed into fine powder. The powder material was dried at 60°C for 3 hours by electric oven. All chemicals were used of analytical grade unless otherwise and the results were depicted as the mean value of the three replicates on dry weight basis.
Determination of moisture contents of Curcurbita maxima seed: Moisture content was determined by the conventional procedure ICOMR [7].
Determination of ash content of Curcurbita maxima seed: Ash content was determined following the method of AOAC [8].
Determination of crude fibre: Crude fibre is the organic residue, which remains after the food sample has been treated under standardized condition with petroleum spirit, boiling dilute sulphuric acid, boiling dilute sodium hydroxide solution and alcohol. The crude fibre consists largely of cellulose together with a little lignin [9].
Determination of lipid content of Curcurbita maxima seed: Lipid content of Curcurbita maxima seed powder was determined by the method of Bligh and Dyer [10].
Determination of total protein: Total protein content of seed of Curcurbita maxima was determined by the micro-kjeldahl method Ranganna [11].
Determination of water-soluble protein: Water-soluble protein content of the Cucurbirta maxima seed was determined following the method of Lowry et al. [12]. The extraction was carried out with distilled water.
Determination of total sugar content of Curcurbita maxima seed: Total sugar content of Curcurbita maxima seed was determined colorimetrically by the anthrone method as described in Laboratory Manual in Biochemistry Jayaraman, [13].
Determination of starch content of Curcurbita maxima seed: The starch content of the Curcurbita maxima seed was determined by the Anthrone method, as described in Laboratory Manuel in Biochemistry Jayaraman, [14].
Determination of minerals: We placed a clean container (dish or beaker) in an oven at 105°C overnight. The container was allowed to cool in a desiccator and weighed, after which the sample was placed into the container and weighed again. The container was placed in an oven at 105°C for 24 hours, after which it was allowed to cool and weighed again. The whole process was repeated until the weight become constant. We store the dried sample in an airtight container and calculated the moisture content in the sample. The sample was grinded in a plant grinder fitted with a suitable screen. If the grinding takes a long time, the sample may absorb moisture. In such a case, it would be necessary to dry the sample again in an oven at 105°C overnight.
Determination of total Nitrogen: Total nitrogen was determined by the Kjeldahl Method, described earlier in the determination of total protein [15].
Determination of Ca, Mg, K, Na, Fe, Mn, Zn, Cu, and P: Organic matter is digested and Ca, Mg, K. Na, Fe, Mn, Zn, Cu, and P are released by digestion with nitric acid. Ca, Mg, Fe, Mn, Zn, and Cu are determined by atomic absorption spectrophotometry.
Determination of Ca, Mg, K, Na and P
Measurement of Ca: 20 ml diluted filtrate was transferred from 50 ml filtrate to a 100 ml volumetric flask. The flask was made up to volume with distilled water and mixed. The content of Ca was measured by atomic absorption spectrometer (AAS). If the reading was higher than the reading of the highest standard solution, made a larger dilution, e.g. 10 ml filtrate into a 50 ml volumetric flask. In this case 1:100 diluted HNO3 had to be added to the volumetric flask to make the total volume of 1:100 diluted HNO3 and filtrate equal to 20 ml.
Measurement of Mg: 5 ml diluted filtrate was transferred into a 50 ml volumetric flask using a pipette. 5 ml LaCl3 solution (pipette) was added and made up to volume with water and mixed. Measure the content of Mg by atomic absorption spectrometer (AAS). If the reading was higher than the reading of the highest standard solution, made a larger dilution, e.g. 2 ml filtrate into a 50 ml volumetric flask, In this case 1:100 diluted HNO3 ml had to be added to the volumetric flask to make the total volume of 1:100 diluted HNO3 and filtrate equal to 5 ml.
Measurement of K and Na: 10 ml diluted filtrate was transferred into a 50 ml volumetric flask using a pipette. The flask was made up to volume with water and mixed. The content of K and Na was measured by flame photometer. If the reading was higher than the reading of the highest standard solution, we made a larger dilution, e.g. 5 ml volumetric flask. In this case 0:100 diluted HNO3 had to be added to the volumetric flask to make the total volume of 1:100 diluted HNO3 and filtrate equal to 10 ml.
Measurement of P: 5 ml diluted filtrate (pipette) was transferred to a 50 ml volumetric flask. Approximately 30 ml of water was added and mixed, again 10 ml ammonium molybdate-ascorbic was also added and it was made up to volume with water and mixed. After 15 minutes, the absorbance was measured on a spectrophotometer at 890 nm, if the absorbance was higher than that of the highest standard solution. The procedure was repeated using a smaller amount of filtrate. In this case 1:100 diluted HNO3 had to be added to the volumetric flask to make the total volume of 1:100 HNO3 and filtrate equal to 5 ml. If the content of P is very high, it is necessary to dilute the filtrate further before the transfer to the 50 ml flask. The dilution is made with water using pipette and volumetric flask. After transferring of 5 ml diluted filtrate to the 50 ml volumetric flask. 5 ml 1:100 diluted HNO3 and water to approx. 30 ml are added. Then 10 ml ammonium molybdate-ascorbic acid is added, the 50 ml volumetric flask is made to volume with water and the absorbance is measured at 890 nm after 15 minutes.
Measurement of Fe, Mn, Zn and Cu: The content of these elements were measured by atomic absorption spectrometer (AAS) directly in the undiluted filtrate.
Extraction of oil
Extraction of oil was done by the method of Southcombe et al. [16]. The powdered seed was placed in a thimble of the soxhlet extractor. The lower end of the soxhlet was attached to the mouth of a round bottom flask containing petroleum ether (b.p. 40°-60°C) as an extraction solvent. The extraction of oil was completed in twelve cycles. The petroleum ether extract so obtained was evaporated under reduced pressure to obtain oil.
Purification of crude oil
Purification of crude oil was done by the method of Ekramul et al [17]. About 100 g of oil was taken in a separating funnel followed by the addition of 100 ml of water, 200 ml of ether and 25 ml of saturated sodium chloride solution. The content of the separating funnel was shaken well and allowed to stand for some time until two distinct layers were separated. Discarding the aqueous layer, the organic layer was again shaken with 100 ml of distilled water and 25 ml of saturated solution of sodium chloride and was allowed to stand. The ether layer was separated and subjected to similar treatment once more. Finally, the resulting ether extract was taken in a conical flask and dried over anhydrous sodium sulfate. The extract was then evaporated by a rotary evaporator at 40°C to get the purified oil.
Determination of specific gravity
Specific gravity of oil was measured by standard test method for specific gravity of oils and liquid fats [18]. Specific gravity of a substance represents how much heavier the substance to the same volume of water at a particular temperature and it may be defined as the ratio of the weight of the definite volume of substance to the weight of the definite volume of water at a particular temperature. The specific gravity bottle was cleaned carefully, dried and weighed when empty. The bottle was then filled with distilled water and holding it in an inclined position to avoid the formation of bubbles. It was then immersed in a constant temperature water bath at 30°C with its stoppered, just outside the level of water. The bottle was removed from the bath, wiped dry with a clean cloth and allowed to stand for some time and then weighed.
The bottle was then emptied, cleaned, dried and filled with the oil; care was taken to avoid the formation of bubbles. The bottle with its content was subjected to similar treatment at 30°C, as was done previously with distilled water and was finally weighed.
Fatty acid composition of oil
Fatty acid composition of the pumpkin seed oil was analyzed by 4500 U-Pye-Unicam gas chromatogram, following the procedure described below [19].
Determination of Iodine value of Pumpkin seed oil
The iodine value of oil was measured by the method Hanus et al. [20].
Determination of saponification value and saponification equivalent
A weighted quantity of the oil was saponified with a known amount of potassium hydroxide, excess of which was determined by titration [21].
Saponification equivalent
Saponification equivalent of a fat or oil is the number of grams of material saponified by one mole of potassium hydroxide.
Determination of acid value and percentage of free fatty acid
The acid value of a fat or oil is the number of milligrams of potassium hydroxide required to neutralize 1 g of the fat or oil. It is a measured the number of free fatty acid presents it [22].
A weighed quantity of material was titrated in a suitable solvent with aqueous sodium hydroxide solution under conditions, which do not saponification of the neutral portion.
Free fatty acid
The percentage of free fatty acid was calculated using the following formula Percent of free fatty acid 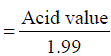
Moisture
Moisture content of Curcurbita maxima was presented in Table 1, the result shows that the Curcurbita maxima seed contains 4.06% moisture. Moisture plays an important part in the growth of trees. Water is indispensable for the absorption and transport of food, to carry out photosynthesis, to metabolize materials and to regulate moisture in plants, as in all other living systems [23].
It contributes as much as to the essential properties of life as do the other constituents like protein, carbohydrate. Moisture is also essential for most of the physiological reactions in plant tissue and in its absence life does not exist [24].
Ash
Ash content of Curcurbita maxima seed is presented in Table 1. Most of the inorganic constituents or minerals are present in ash. The seed was found to contain about 3.80% ash, which was lower than the values 4.62% for Teramnus labialis seed Viswanathan et al., totally [25].
| Parameters | Moisture | Ash | Crude fibre | Total Lipid | Total Protein | Total Soluble Protein | Sugar | Starch |
|---|---|---|---|---|---|---|---|---|
| Content % | 4.06 | 3.8 | 2.91 | 36.7 | 34.56 | 18.1 | 1.08 | 2.15 |
Table 1: Nutrient composition of Cucurbita maxima seed.
Crude fibre
Fibre is an important component of many complex carbohydrates. It is always found only in plants particularly vegetables, fruits, nuts and legumes. As shown in Table 1, crude fibre content of the cucurbita maxima seed was found to be 2.91% which was lower than the of 4.68- 6.92g% for cassia hirsute seed [26].
Lipid content
Lipid is more useful in animal body. Fat serves as efficient source of energy and insoluble material. Dietary fat helps in the absorption of fat soluble vitamins, lipoproteins are important cellular constituents. Lipids are essential components of cell membrane, source of metabolic energy for cell maintenance, reproduction and embryogenesis in insect.
As shown in Table 1, Curcurbita maxima seed contains total lipids 36.70 g% which is much higher than the values 4.00 g% of Cassia fistula seeds Zaka, [26] and 9.58 g% of Xylopiaaethiopica seeds bar minas, [27]. From the results, it may be concluded that the Curcurbita maxima seed is an important source of lipid.
Total protein
Total protein of Curcurbita maxima seed was quantified to be 34.56 g% which is higher than the value of 7.12 g% for Attalea cohune seed (Williams), and the value of 27.50 g% for Viciafaba seed Vetter, [28].
Total soluble protein
The result for protein content of Curcurbita maxima seed obtained from our experiment is given in Table 1.The result shows that the Curcurbita maxima seed contains 18.10% total soluble protein. The protein constituents are of primary importance not only as component of nuclear and cytoplasmic structures, but also as complement of enzyme involved in metabolism during growth, Percent of protein content (g per 100 g of Curcurbita maxima seed) (Figure 1).
Total sugar content
Carbohydrate plays an important role on the physiological activities of the plants. Glucose and glycogen serve as important source of energy for vital activities. Some carbohydrates have highly specific function.
The experimental data is presented in Table 1, from this it was found that the total sugar content was estimated to be 1.08 g%, which is lower than that for Brazil nuts 1.9 g% Williams, [29] (Figure 2).
Starch content of C. maxima seed
Starch is the most important source of carbohydrate in human diet. It is found in most plants, particularly in the seeds, where it serves as the nutritional reserve of carbohydrate. Starch content was determined and found to be 2.15g% (shown in Table 1), which is lower than that of 5.25 g% loofah seeds Devine, [30].
Minerals content of C. maxima seed
Minerals are inorganic substance required by the organism in very small amount for their growth and maintenance of functional activity. Food and vegetables are the important source of mineral for human beings and exist in food as organic and inorganic combination. In foods mineral elements are present as salt. They combined with organic compound, e.g. iron in hemoglobin. Minerals are required for the teeth and bone formation. Minute amount of mineral elements are constituent of various regulatory compounds such as, vitamins, enzymes and hormones. For example, some enzymes require calcium for their activity as lipases and succinate dehydrogenases. Iron requiring enzymes are ferredox in catalase, indophenol oxidase, aldehyde oxidase etc. the mineral elements present in the intra and extra cellular fluid maintained water and acid-base balance. They regulate transmission of impulses and contraction of muscles. The deficiencies of minerals create many diseases in human beings. The amount of N, Ca, Mg, K, Na, Fe, Mn, Zn, Cu, and P present in the C. maxima seed is shown in Table 2.
| Parameters | N | P | Na | K | Ca | Mg | Fe | Cu | Zn | Mn |
|---|---|---|---|---|---|---|---|---|---|---|
| % | Cmol/kg | PPm | ||||||||
| Amount | 5.53% | 0.71% | 5 | 20 | 4 | 349 | 290 | 70 | 40 | 18 |
Table 2: Amount of Mineral in Cucurbita maxima seed.
Determination of specific gravity
Specific gravity of fats and oils do not vary as a general rule to an extent, which makes this property useful in discriminating between one to another the specific gravity of practically all fats or oils lie between 0.900 and 0.950 [31].
Specific gravity of the oil was determined by strand method and was found to be 0.9412 at 31°C.
Fatty acid composition of the oil
Fatty acid analysis of the pumpkin seed oil by GLC was carried out after transesterfication of the glycerides to their methyl esters. The fatty acid composition of the oil samples is presented in Table 2 and gas chromatograms are shown in Figure 3. From the Table 2 it is found that pumpkin seed oil contains the highest amount of oleic acid 40.58% while stearic acid, palmitic acid and Linoleic acid content are found to be 27.06, 17.39 and 14.97%, respectively. The GLC data also indicates that the pumpkin seed oil contains higher amount of unsaturated acid fatty acids 55.55%, while saturated fatty acid is found to be 44.45%.
From the present investigation it may be suggested that pumpkin seed oil contains essential fatty acid viz., Linoleic acid, which is useful in human body and properly refined oil may also be used as edible oil.
Determination of Iodine value
Iodine value gives the estimation of the amount of unsaturated fatty acid in the triglyceride molecule of fat and oil. The iodine value was measured by the Hanus method and was found to be 114.33, which is lower than the values of (136.9-137.9) [32] and (137.1-138.0) for tobacco seed oil, and also to the value of 173 for Ocimum pilosun seed oil. In general, the higher degree of unsaturation i.e. the higher iodine value, the greater is the liability of the oil or fat to become rancid by oxidation. Therefore, seed oil in pumpkin has higher tendency to become rancid by oxidation.
Saponification value and saponification equivalent
Saponification value is inversely proportional to the average molecular weight or chain length of the fatty acid present in the fat or oil. Oils and fats consisting largely of C18 fatty acids generally have saponification value around 290.80, indicating the presence of appreciable quantity of higher fatty acids.
The saponification of pumpkin seed oil was determined to be 193.73 whereas the saponification equivalent was calculated from saponification value to be 289.58. The saponification value of this seed oil is higher than those of (189.20-190.50) for the tobacco seed oil and (186.37-188.40) for Cassia fistula seed oil.
These comparatively high saponification values indicate the presence of low proportion of lower fatty acids. The present result also indicates that pumpkin seed oil contains high proportion of higher chain fatty acid than those of tobacco oil and cassia fistula seed oil.
Acid value and percentage of free fatty acid
Acid value is measured of the free fatty acids present in the oils or fats and differs from the determination of the “free fatty acids” only in the interpretation and manner of expression. The assumption usually being made in a calculation that the acid have a molecular weight equal to that of oleic acid. A high acid value may indicate a higher tendency to become rancid. A high percentage of free fatty acid (above 1.15%) indicates that the oil is not suitable for edible purpose. In present investigation, the acid value of the pumpkin seed oil was found to be 0.516. The percentage of free fatty acid of seed oil was calculated from acid value and found to be 0.2646%. Hence the acid value of this seed oil was much lower than the value (1.90-2.30) for tobacco oil. The present result suggests that pumpkin seed oil is suitable for edible purposes, as the amount of free fatty acid was found to be lower than 1.15%.
Cururbita maxima, commonly known as Mistikumra, is cultivated all the districts of Bangladesh to satisfy nutritional requirements. Having a database of the analysis of cultivated plants available in the region would be of value to educator and public health officials positioned to provide dietary advice to the food stressed population.
From the nutritional analysis, it was found that the seeds of Curcurbita maxima are rich in lipid 36.70%, protein 34.56% and crude fibre 2.91%. Therefore, it may be used as potentially attractive source of lipid, protein and crude fibre. In addition, the seed is a good source of minerals like nitrogen, phosphorus, sodium, calcium, copper, zinc, magnesium, potassium, and iron that are important for our health. The seed may also be used as fertilizer since it contains 5.53% nitrogen.
Curcurbita maxima seed yields 12% of oil. Analysis of seed oil implied higher degree of unsaturation possessing comparatively high iodine value (144.33). High degree of unsaturation makes it suitable for the use as valuable drying agent, and lower free fatty acid content indicates suitability of the oil for probably edible purpose. Results of fatty acid composition reveal that Cururbita maxima seed oil is a rich source of linoleic acid, which is useful in human body (Table 3).
| Retention time | Fatty acids | Relative percentage (%) |
|---|---|---|
| 14.82 | Palmitic acid (C15H31COOH) | 17.39 |
| 17.38 | Linoleic acid (C17H31COOH) | 14.97 |
| 17.9 | Oleic acid (C17H33COOH) | 40.58 |
| 18.1 | Stearic acid (C17H35COOH) | 27.06 |
Table 3: Fatty acid composition of Cucurbita maxima seed oil.
On the basis of the above discussion, it can be concluded that the seed of pumpkin can be used as an important nutritional source for the people of Bangladesh or in the area present malnutrition. On the other hand, the oil can be utilized in paint, varnish and ink industries. Again, the seed oil can be suitable for human consumption after proper refining, and may provide a relief from the oil crisis.
There is no conflict of interest
This study was carried out in the Department of Biochemistry and Molecular Biology, Rajshahi University and supported by the University Grant Commission (UGC), Bangladesh.