
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)
Background: OCT-A is a new imaging modality that provides the opportunity for noninvasive characterization and quantification of the microvasculature in wet AMD.
Purpose: The purpose of our study is to outline the different OCT-A characteristics of neovascular membranes in AMD in regard to their correlation with the effect of treatment and prognosis.
Methods: In our study 42 patients with wet AMD were enrolled. They were divided in 3 groups: 25 with Type 1 membranes (under RPE), 11 patients with Type 2 membranes and 6 with Type 3 RAP lesions. They all underwent a complete ophthalmological examination including VA, fundus photography, structural OCT (Rtvue, Optovue) and OCT-A (Angiophlex, Zeiss). All of the patients were treated with aflibercept (Eylea)-3 injections. They all were evaluated after the third injection.
Results: In the group of patients with type 1 CNV usually a medusa shaped neovascular complex with afferent vessel, originating in the choroid was observed on the OCT-A. In 9 cases the vessels were very rough and larger than in the general group. In the type 2 CNV group we visualized a neo-vascular network that grows from the choroid vasculature traverses the RPE-Bruch’s membrane complex into the sub-retinal space. These structures usually had glomeruli like shape. Type 3 CNV is seen on OCT-A as retinal-choroid anastomoses originating in the deep capillary plexus of the retina. The majority of patients from all groups improved VA and retinal thickness after the application of Eylea. Only in 2 cases no change was seen. Those were mainly cases with Type 1 CNV and with larger caliber of the vessels.
Conclusion: OCT angiography is a new imaging modality capable of revealing the morphological structure of Type 1, Type 2, and Type 3 neovascularization in exudative AMD. Visualizing the microarchitecture of the CNV membrane offers the prospect to evaluate the possible responses to therapy. Our results indicate that type 1 lesions, composed of larger, mature vessels are less responsive to anti-VEGF therapy and are with poor prognosis.
OCT-A; AMD; Prognosis; Therapy
Optical Coherence Tomography (OCT) is a relatively new noninvasive method for diagnostics of the retina and optic nerve [1,2]. It is a technology similar to B sonography but instead of ultrasound it uses light with wavelength of 840 nm. It is a novel way of diagnosing retinal structures, called by some people “optical bio microscopy” or “in vivo microscopy” of the eye. That is because it gives us information about the structures similar to that gained by histological slides [3-5]. It can be implicated that Optical Coherence Tomography (OCT) technology allows the acquisition of cross-sectional images of the retina with semi histologic resolution. It permits to define the location and nature of the changes in the retina and adjacent structures and objectively evaluates the thickness of the retina and surrounding structures. These capabilities allow detection of newly emerging fluid and/ or intraretinal or subretinal tissue and tissue below the retinal pigment epithelium. This is of extreme importance in evaluating the progression of AMD patients. All the above outlines the great practical application of this methodology for the diagnostics and progression of multiple ophthalmological diseases such as glaucoma, retinal disorders, AMD etc.
If we look historically on the way the OCT technology developed, we can mention the emergence of the OCT machines with Time Domain OCTs in 2006. Later it was surpassed by the Spectral domain OCT and the last emerging technology is the Angio- OCT-2014 [6-8]. Modern technology, especially newer versions of Optical Coherence Tomography (OCT), that incorporates Spectral Domain (SD) technology has immensely improved the ability to evaluate retina and AMD changes [9-11]. SD technology provides higher scan resolution and scan speed compared to conventional Time Domain (TD) OCT and it enables differentiation of the different layers in the retina including RPE and photoreceptors [12]. The OCT images are presented in a color (or gray) scale based on the different reflectivity of the tissue structures.
More concisely, tissues that reflect more light or disperse more light are shown in red and white, respectively, while the ones that reflect or disperse less light are shown in blue and black. Tissues that moderately reflect light are shown as green or yellow. It should be noted that the color shown in the images represents the optical properties of the tissues and not the tissues themselves. Therefore, the image is not real but represents the true dimensions of the measured structures [13-15]. SD-OCT provides more detailed images of intraretinal, subretinal fluid when compared to timedomain technology, leading to higher and earlier detection rates of neovascular AMD activity. Improvements in image analysis and acquisition speed make it important for decision-making in the diagnosis and treatment of retinal diseases. The main advantages of OCT diagnostics in retinal diseases can be concluded in the following way:
• It is noninvasive technology.
• Available and easy to do.
• Gives us the opportunity to evaluate minimal quantities of fluid in the retina-retinal quantitive analysis.
• Gives quantitative retinal analysis as well as morphological evaluation of the neurosensory retina.
• Evaluation of the progression of the disease.
All the above mentioned advantages of OCT over other methods make it diagnostic of choice for macular diseases.
Optical Coherence Tomography Angiography (OCTA) is the newest non-invasive imaging technique that employs motion contrast imaging to high-resolution volumetric blood flow information generating angiographic images in a matter of seconds. OCTA compares the decorrelation signal (differences in the backscattered OCT signal intensity or amplitude) between sequential OCT b-scans taken at precisely the same cross-section in order to construct a map of blood flow. Axial bulk motion from patient movement is eliminated so sites of motion between repeated OCT b-scans represent strictly erythrocyte movement in retinal blood vessels [16-19]. OCTA requires higher imaging speeds than most currently available OCT systems can provide in order to obtain a densely sampled volume. Conventional OCT device scanning speeds would result in too much trade-off between decreased field of view, lower image quality, and greatly increased scanning time.
OCT A is often compared to Fluorescein Angiography (FA). In contrast to OCT A, FA is an invasive test that requires intravenous administration of dye and imaging up to 10-30 minutes [15]. It provides two-dimensional image sets that allow for dynamic visualization of blood flow with a wide field of view. Therefore, patterns of dye leakage, pooling, and staining can be appreciated and are well-documented in the literature [20]. FA remains the gold standard for the detection of choroid neovascularization. However, retinal pathology can be obscured by this leakage as well as hemorrhage or media opacities, and localization of the depth of the lesion and size delineation of neovascularization can be difficult due to dye leakage and poor stereopsis. On another hand FA is an invasive, relatively expensive and time-consuming procedure, which although considered safe, poses risks ranging from nausea to allergic reactions, including anaphylaxis in rare instances [21].
OCTA in comparison is a non-invasive technique that acquires volumetric angiographic information without the use of dye. Each three-dimensional scan set takes approximately six seconds to obtain. The en-face images (OCT angiograms) can then be scrolled outward from the Internal Limiting Membrane (ILM) to the choroid to visualize the individual vascular plexus and segment the inner retina, outer retina, choriocapillaris, or other area of interest [22,23]. OCT angiograms appear to be higher resolution than the currently available FA/images, and several studies deduced [24] that they were at least equivalent in showing important vascular detail [25]. Use of the montage technique allows for a larger field of view much like FA while maintaining this improved resolution. OCTA provides flow information at a fixed point in time. Although leakage is not appreciable, exact delineation and size measurements can be performed for pathology such as CNV [26]. OCTA provides both structural and functional (i.e., blood flow) information in tandem. Both the retinal and the choroid microvasculature can be visualized using OCTA while FA is used for seeing the retinal vessels and ICGA is more ideal for imaging the choroid. Using precise software programme we can obtain a map of the retinal microcirculation. Segmentation of the retina is made in OCT A dividing the retina into 3 major zones-superficial plexus, showing the retinal vasculature between ILM and IPL, deep retinal plexusshowing the vasculature between IPL and OPL. The deep plexus vasculature is undetectable for FA. The last zone seen on the OCTA is the avascular zone situated between OPL and RPE. Although a novel technology OCTA has shown to be an extremely useful in the diagnostics of AMD and types of neovascular membranes. However how useful the OCTA images can be in predicting the course of the disease remains still unclear. The aim of our study is to outline the different OCT-A characteristics of neo-vascular membranes in AMD in regard to their correlation to the effect of treatment and prognosis.
The clinical study included a group of 42 patients with wet AMD. In that group 25 were with Type 1 membranes (under RPE), 11 patients with Type 2 membranes and 6 with Type 3-RAP lesions. The Medical Ethics Committee of the Military Medical Academy Sofia approved the study protocol and guaranteed that all the rights of the patients are protected and they consented to providing their data for the clinical study. The study followed all the official guidelines and regulations implied for conducting studies and research activities. All patients were treated at the same institution. They all underwent a complete ophthalmological examination including VA, fundus photography, Fluorescein Angiography (FA), structural OCT (Rtvue, Optovue) and OCT-A (Angiophlex, Zeiss). We used OCTA 3 × 3 mm as well as 6 × 6 mm.
All of the patients in the 3 different groups were treated intravitreally with Eylea (Aflibercept) in dose 2 mg (0.05 ml). The mean number of intravitreal injections was 7 (SD 3.4). They all were evaluated one month after the third injection. The OCTA images were analyzed with image analyzer for the presence and characteristics of the neovascular membranes.
All the patients were followed for a period of one year. The injections were done following the Treat and extend protocol. In that scheme the pateints had 3 initial loading doses every 4 weeks and later on in patients without activity of the disease the injection interval was extended with 2 weeks. The shortest interval in our patients was 4 weeks.
Statistical analysis was performed using a commercially available statistical software package 125 (SPSS for Windows, V.25.0, IBM-SPSS, and Chicago, IL).
In the group of patients with type 1 CNV usually a medusa shaped neovascular complex with afferent vessel, originating in the choroid was observed on the OCT-A. In 9 cases the vessels were very rough and larger than in the general group.
In the type 2 CNV groups we visualized a neovascular network that grows from the choroid vasculature traverses the RPE-Bruch’s membrane complex into the subretinal space. These structures usually had glomeruli like shape.
Type 3 CNV is seen on OCT-A as retinal-choroid anastomoses originating in the deep capillary plexus of the retina. The majority of patients from all groups improved VA and retinal thickness after the application of Eylea. Only in 2 cases no change was seen. Those were mainly cases with Type 1 CNV and with larger caliber of the vessels.
The study included wet AMD patients divided into 3 subgroups. AMD patients with Type 1 membranes (under RPE) consisted of 25 patients (9 men, 16 women) with a mean age of 72.9 ± 10.8 years (median: 70.2 years; range: 57.7-88.5 years). The Type 2 AMD patients group comprised of 11 patients (6 man and 5 women) with a mean age of 81.11 ± 12.3 years (median: 87.0 years; range: 67.1-89.3 years). The last groups were the 6 patients with RAP (1 man, 5 women) with a mean age of 68.9 ± 10.3 00 years (median: 68.2 years; range: 57.7-68.5 years). All the groups were examined with Fluorescein angiography (FA), structural OCT and OCTA. According to the OCTA the neovascular membranes can be divided into 3 main types such as:
• Type 1 CNV situated under the RPE.
• Type 2 CNV situated above the RPE in which the vessels originate from the choroid and transverse the RPE.
• Type 3 CNV with retino-choroidal anastomoses coming from the deep retinal plexus and corresponding to RAP lesions.
With the use of OCTA and the whole complex of diagnostic tools we were able to study the structure of neovascular membranes of our patients.
In the first group of 25 AMD patients we had a Type 1 neovascular membrane, in which the new vessels originated from the choroid and transverse the Bruch’s membrane and were located underneath the RPE.
In 50% of the cases (12) the neo-vascular membranes were with the shape of “medusa” or coral-form with multiple radial branching vessels, emerging from main feeding one (Figure 1A).
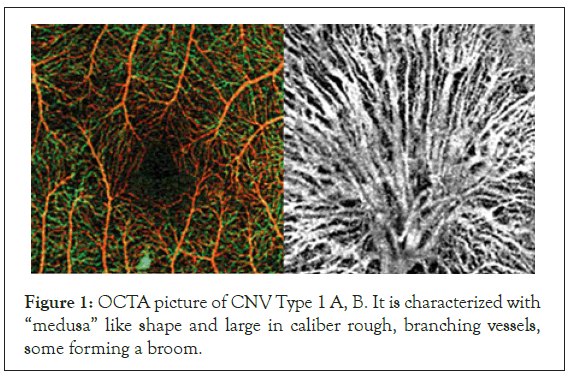
Figure 1: OCTA picture of CNV Type 1 A, B. It is characterized with “medusa” like shape and large in caliber rough, branching vessels, some forming a broom.
In some of the cases it was very difficult to detect the neovascularistion as it was situated underneath the RPE. Only in the images trough the choroid we were able to detect the emerging new vessels and see their structural characteristics. All other segmentations of OCT A gave normal images.
In 7 patients-2% of this group--the new vessels were very large in caliber, rough looking with numerous branches, forming a network of small capillaries or looking like a broom. These types of CNV were usually prone to progression and they were the ones that did not respond to the therapy (Figure 1B). In about 1% of the patients with CNV Type I the vascular membranes lacked distinct smaller vessels, although we were able to outline the big feeding one.
The Type 2 AMD patients group comprised of 11. The lesions emerged from the choroid, traversed the RPE and formed a membrane located between the RPE and neurosensory retina. These are the classic type CNVs found in the FA. These lesions had well outlined borders on the OCT-A and typical shape. In 7 patients the OCTA characteristics were that of “glomeruli” like shape with multiple branching little vessels with different hypo reflectivity (Figure 2).
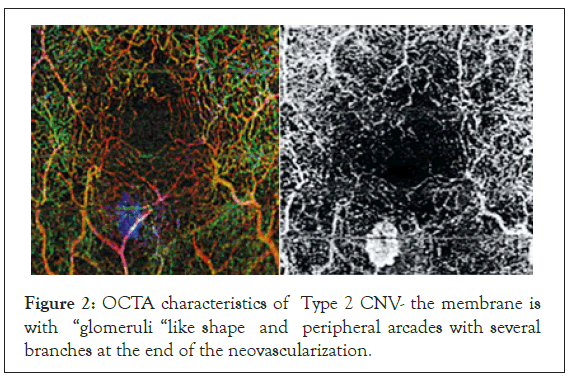
Figure 2: OCTA characteristics of Type 2 CNV- the membrane is with “glomeruli “like shape and peripheral arcades with several branches at the end of the neovascularization.
In some of the cases peripheral arcades with several branches at the end on the neovascular membrane were seen on the OCTA images. They were considered to be a hallmark of activity and of further progression of the neovascular membranes. The last groups were the 6 patients with RAP (1 man and 5 women). In the RAP lesions the new vessels originally emerged from the deep retinal plexus. OCT-A gave us the opportunity to see the lesions in the superficial and deep retinal plexus and its junctions with the vessels of the choroid, where they formed retino-choroidal anastomoses (Figure 3). CNV type 3 was characterized by a tuff shaped high–flow vascular network arising from the deep capillary plexus in the outer retinal layer. In all eyes, a feeder vessel was seen connecting the CNV with the deep retinal capillary plexus. About 80% of lesions we found were associated with pigment epithelial detachment.
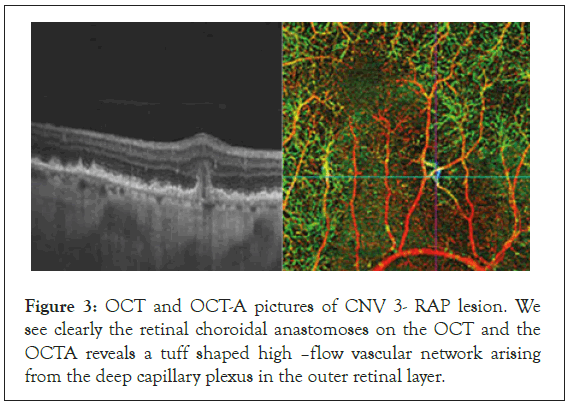
Figure 3: OCT and OCT-A pictures of CNV 3- RAP lesion. We see clearly the retinal choroidal anastomoses on the OCT and the OCTA reveals a tuff shaped high –flow vascular network arising from the deep capillary plexus in the outer retinal layer.
All of the patients in the 3 different groups were treated intravitreally with Eylea (Aflibercept) in dose 2 mg (0.05 ml). The mean number of intravitreal injections was 7 (SD 3.4). All the patients were followed for a period of one year. The injections were done following the Treat and extend protocol. In that scheme the pateints had 3 initial loading doses every 4 weeks and later on in patients without activity of the disease the injection interval was extended with 2 weeks. The shortest interval in our patients was 4 weeks.
The mean VA of our patients was 0.1 (SD 0.2) for the three groups. After the application of the treatment the VA increases averedgedly with 6 ± 2 letters by the end of the year follow up. The mean improvement of the VA was up to 0.3 (SD 0.4; P<0.001). We established also significant reduction of the central retinal thickness. From initial mean retinal thickness of (400 ± 130 μm) by the end of the year the reduction of the retinal thickness was up to (294 ± 75 μm).
In the 7 patients from the first group with typical very large vessels of the membranes on the OCTA no improvement of the VA has been found. The mean VA of 0.1(SD 0.2) remained unchanged and even in 2 patients reduced to 0.05. The central retinal thickness was very slightly changed after the treatment from (395 ± 110 μm) up to ( 300 ± 75 μm ). We can say that particular subgroup was resistant to therapy.
However overall, all patients showed decrease in the density of the small branching vessels of the neovascular membranes after the anti-VEGF therapy. In this subgroup that decrease in the density was the smallest but still existed.
Our study gave us the opportunity to outline the main criteria on OCTA showing and predicting progression, which can be an indicator for starting anti-VEGF treatment. Patients with neovascular membranes which on the OCT A are with “medusa” like shape or lace with rough large vessels and numerous branching capillaries have been the ones with quick progression and resistant to therapy. Another risk factor for progression is the presence of anastomoses between the vessels. The new active vessels are usually large in caliber, branching and forming a network. In the 3 groups of patients we had 7 such patients who progressed further in the period of 1 year. The RAP patients were also difficult to treat and they preserved the initial visual acuity but with maximal number of injections.
OCTA is a novel, modern technology, which is about to increase its range and abilities in the future. In recent years, it is the newest imaging modality to simultaneously obtain structural images of the retina as well as assess blood flow within the retinal and choroid without the use of intravenous agents [27,28]. Optical coherence tomographic angiography provides vascular imaging via motion contrast processing of decorrelation signals. It is a diagnostic tool which enables us to gain new information about the types of CNV in AMD patients. It gives us a glimps on the morphology of the neovascular membranes and their peculiar types and shapes [29]. CNV has been classified based on the findings of OCTA into 3 different types: type I CNV, type II CNV, and type III CNV.
Lots of authors [29] have studied the OCT a shape of neovascular membranes. Sarraf et al. described the morphologic characteristics of type I CNV lesions on OCTA (RTVue XR AvantiAngioVue). In their study of 33 eyes with AMD, they described 2 distinct appearances of type I membranes: a “medusa” form (about 55% of the lesions) with vessels radiating in all directions from a large main feeder vessel and a “sea fan” form (about 25% of lesions) where most of the smaller vessels radiated from one side of a large feeder vessel.
Their data [29,30] corresponds completely to our findings that show that the majority of our patients with CNV type one had also “medusa” or coraciiform shape (about 50% of the lesions) with multiple radial branching vessels, emerging from main feeding vessel. However what we encountered was that in 2% of the new vessels were very large in caliber, rough looking with numerous branches, forming a network of small capillaries or looking like a broom. These types of pictures on the OCTA were connected with poor prognosis and resistance to the applied therapies. Our data show that there is a correlation between OCTA pattern and the result of the anti-VEGF therapy. It is easy to assume that probably the bigger the vessels in the structure the smaller the probability for anti-VEGF agents to be able to cause regression and occlusion in such vessels. Even though we expected to find a correlation between this particular OCTA type and the occurrence of RPE detachment or RPE atrophy no such data was found. Overall, there was no correlation between the morphologic pattern of the lesions and the presence of RPE detachment or RPE atrophy.
However unlike other authors [30] the vessel density of the small branching vessels was notably reduced after the anti-VEGF therapy in CNV type I membranes.
CNV Type 2-3 was described by Souied et al.[27] who revealed their OCTA features and morphological patterns on SD-OCTA. They saw the neovascular membranes in CNV 2 type as “medusa shaped” lesions or “glomerulus-shaped” most of which had a larger feeder vessel extending from the choroid.
That comes also in support to our findings stating that the majority of the neovascularisations of the CNVs Type II emerged from the choroid, Trans passed the RPE and was situated between the RPE and neurosensory retina. In some of the cases peripheral arcades with several branches at the end of the neovascular membrane were seen on the OCTA images. Membranes in which no branching vessels were found were less active and unlike of progression.
Sarraf and al. have shown the characteristics of type III CNV lesions with OCTA. In their study of 29 eyes with type III wet AMD; a distinct neo-vascular membrane could be seen in 10 eyes (34%) on OCTA. The lesions were active on OCT and appeared as small tufts of high-flow vessels in the outer retinal layer. Their data also corresponds with our findings in which CNV type 3 were characterized by a tuff shaped high-flow vascular network arising from the deep capillary plexus in the outer retinal layer. In all eyes, a feeder vessel was seen connecting the CNV with the deep retinal capillary plexus. About 80% of lesions were associated with pigment epithelial detachment. Generally RAP lesions were more difficult to treat and to respond to therapy [31-33].
In our study we found a correlation between morphology of the membranes and the response to therapy and progression of the disease. OCT A gave us an opportunity to outline the risk characteristics for resistance to therapy:
• CNV type I with “medusa” like shape, large rough branching vessels, with numerous peripheral branching are more likely to be resistant to therapy
• RAP lesions showing one large feeder vessels connecting the CNV with the deep capillary plexus were also considered with poor prognosis.
In conclusion OCT angiography is a new imaging modality capable of revealing the morphological structure of Type 1, Type 2, and Type 3 neovascularization in exudative AMD. Correlation between morphological characteristics on OCT A and therapeutic response enables us to predict the further development of the disease. Future investigations with OCTA to examine the natural history, progression, and response to treatment in AMD may ultimately lead to a better understanding of the pathogenesis of this disease and improved patient outcomes.
Funding
None
Financial disclosures
None.
Availability of data and material
All data will be made available upon request.
Ethics approval/Consent to participate/Consent for publication
The Medical Ethics Committee of The Military Medical Academy Sofia has approved the study protocol and had waived the necessity to obtain the written consent of the individual patients. All patients by admitting in the hospital give their consent their data to be used for clinical trials.
Code availability
The statistical analysis was performed using a commercially available statistical software package (SPSS for Windows, V. 25.0, IBM-SPSS, and Chicago, IL).
Citation: Vidinova C, Antonova DP, Guguchkova PT, Vidinov KN (2021) OCT-A Characteristics of Neovascular Membranes in Wet AMD-Possible Correlation to the Prognosis. J Clin Trials. S12:004.
Received: 08-Jul-2021 Accepted: 22-Jul-2021 Published: 29-Jul-2021 , DOI: 10.35248/2167-0870.21.s12.004
Copyright: © 2021 Vidinova C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.