Pediatrics & Therapeutics
Open Access
ISSN: 2161-0665
ISSN: 2161-0665
Research Article - (2022)Volume 12, Issue 6
Background: Globally, the number of children with Attention/Hyperactivity Disorder (ADHD) counts approximately 3.4%. One of the most common ways of treatment is the intake of psychostimulants. Hyperactive children can show significantly lower serum levels of omega-3 fatty acids, or even an acceleration of omega-3 fatty acid catabolism, compared to control groups without hyperactivity disorder. Especially Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) are essential for children’s brain growth and function, thus their lack is assumed to influence the progress of the disorder negatively.
Methods: A PubMed search was conducted with key terms “omega-3 fatty acids” OR “children” OR “attention/ hyperactivity disorder”. The search strategy included meta-analyses, randomized controlled trials, clinical trials, observational studies, reviews and book chapters.
Conclusions: Due to the fact, that medication with psychostimulants is associated with several harmful secondary effects, this review presents the current knowledge regarding the impact of omega-3 fatty acid supplementation to alleviate ADHD-symptoms in children.
Attention/hyperactivity disorder; Children: Omega-3 fatty acids; Supplementation
Approximately 3.4% of children are diagnosed with Attention/ Hyperactivity Disorder (ADHD), globally. Prevalences in Europe are comparatively low, whereas the United States represent up to 10.6% of children with ADHD-symptoms [1,2]. The disorder shows symptoms like inattention, physical hyperactivity and impulsivity. As these behavioral problems are partly common in children, in ADHD affected children they are more intense and longer lasting. The symptoms emerge mainly in unpleasant or for the child uninteresting situations. When children perceive activities or situations in a positive way, they rarely show behavioral disorders [3]. ADHD is a heterogenous and multifactorial disease, where approximately 2/3 of ADHD-diagnosed children show complementary comorbidities like anxiety disorders and depression. Psychostimulants such as methylphenidate and amphetamines are routinely used for ADHD medication [4]. Up to 50% of children treated with psychostimulants no longer show any ADHD-symptoms. However, due to the fact that intake of psychostimulants can cause serious side effects, this kind of therapy should not be the first-line ADHD-treatment. Only if behavioral therapies and interventions by psychologists as well as further non- drug treatment methods fail, psychostimulant treatment should be indicated [5,6]. Various studies show a reduction of ADHD- symptoms by the supplementation of omega-3 fatty acids. Especially Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) show positive impact in children with ADHD-symptoms [5,7- 10]. Docosahexaenoic Acid (DHA) is a component of nerve cell synapses and it is involved in signal transduction in the brain [11]. Furthermore, it increases membrane fluidity in the brain and is involved in neurotransmitter processes [8]. Eicosapentaenoic Acid (EPA) also promotes signal transduction and supports cell growth as well as gene expression [12]. Compared to the intake of psychostimulants, omega-3 fatty acids rarely show any side effects [6].
The objective of this manuscript is to review the current state of research concerning the impact of omega-3 fatty acid supplementation in children with ADHD-symptoms. The extent of symptom reduction in children with ADHD-symptoms by supplementation of omega-3 fatty acids is discussed and dietary recommendations concerning the intake of omega-3 fatty acids in natural foods are given.
ADHD´s etiology and experimental data of therapeutic treatment options
The etiology of ADHD is subject of intense research, as the disorder identifies multifactorial triggers [13]. Central nervous system disorders, genetics, neuropsychological deficits as well as neurobiological and psychosocial factors may be involved in the disorder´s pathogenesis. From a neurobiological point of view, a deficit of the neurotransmitters dopamine and norepinephrine seems to be the cause of ADHD. A lack of these neurotransmitters results in a disturbed information transfer within the neural network. Dopamine and norepinephrine are involved in the regulation of impulsive and hyperactive behavior. A low level of these neurotransmitters is associated with an increase in impulsivity and hyperactivity. The disorder´s pathogenesis, involving already investigated aspects of ADHD etiology, is shown in Figure 1 [5,14]. Furthermore, nutrition may also play a significant role in etiology and its process [2,6]. An unbalanced diet can lead to a shortage of macro and micronutrients, which might cause a disturbed maintenance of body functions and mental performance [15]. Certain studies discuss the relationship between the deficiency of Polyunsaturated Fatty Acids (PUFA) in the body and ADHD symptoms [6,14,16]. Shortage of PUFA as well as other nutrients might be a trigger for ADHD genesis [11]. Long-Chain Polyunsaturated Fatty Acids (LCPUFA), like omega-3 fatty acids, promotes the release of dopamine, serotonin and norepinephrine in the brain. Furthermore, they are involved in the regulation of gene transcription and stabilize membrane structures and their fluidity [9,14]. Not only a shortage of nutrients, but also the intake of certain dietary supplements such as preservatives, artificially produced food colors or water contamination with toxic substances like lead may be involved in the pathogenesis of ADHD [6,15,17].
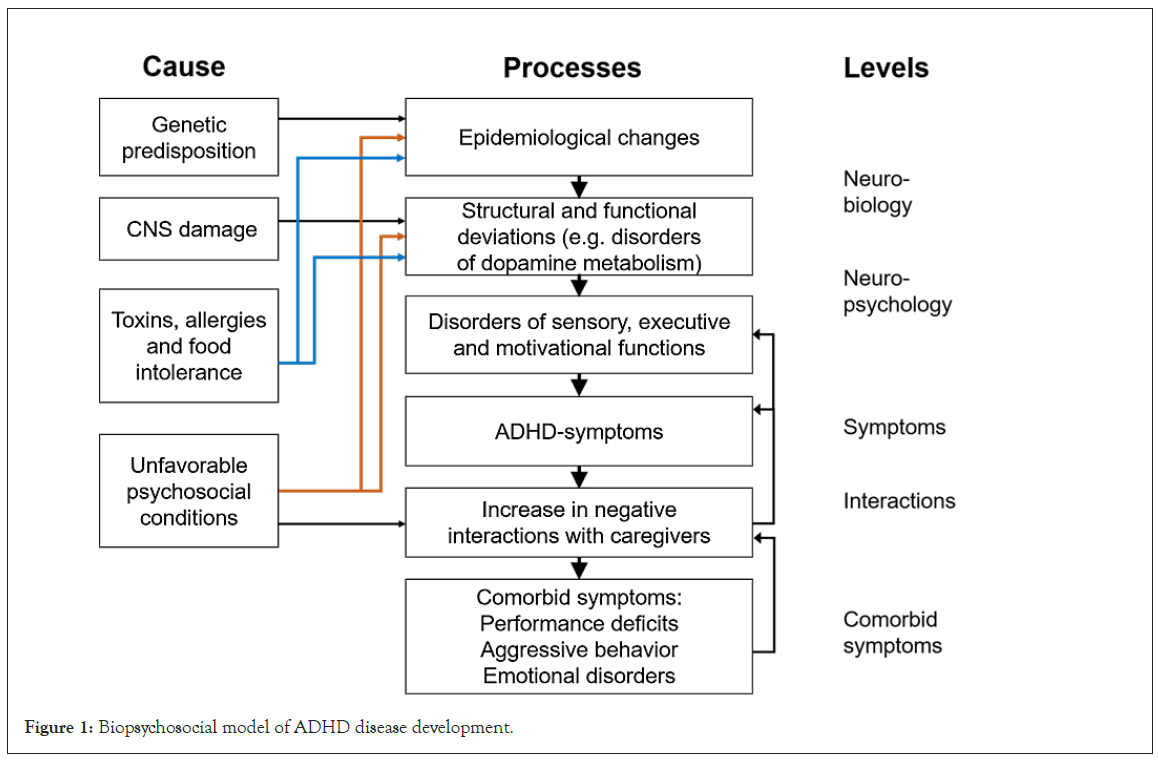
Figure 1: Biopsychosocial model of ADHD disease development.
Psychostimulants such as methylphenidate and amphetamines are routinely used for medication of ADHD. These drugs increase the release of synaptic dopamine in the brain, which results in a reduction of ADHD-symptoms as well as partially reduced comorbid behavioral disorders. Up to 50% of children treated with psychostimulants no longer show any ADHD-symptoms. However, the intake of psychostimulants can cause serious side effects like an increased heart rate, hypertension, tachycardia, increased insomnia, drowsiness, growing feelings of sadness up to depression and also growth disturbance [5,15]. Psychostimulant medication might also lead to malnutrition and weight loss of the child, due to drug reactions causing for example inappetence, constipation, diarrhea, nausea, stomatitis or abdominal pain [15]. Approximately 15 to 30% of children with ADHD do not respond to psychostimulant treatment, thus this kind of treatment is not reasonable in this case. Exclusive psychostimulant therapy should not be the first-line ADHD-treatment. Only if possible behavioral therapies and interventions by psychologists as well as further non- drug treatment methods fail, this form of medication should be indicated [5].
The role of omega-3 fatty acids
Various studies show a reduction of ADHD-symptoms by supplementation of the omega-3 fatty acids DHA and EPA over a minimum period of three months [5,9,10]. DHA represents high levels in the human brain. Approximately 25% of the brain's dry matter consists of DHA and further fatty acids [6,15], which indicates their importance as an essential part of the brain. Due to the significance of adequate PUFA intake in the phase of brain growth, this review analyses several studies regarding the spectrum of symptom reduction in children with ADHD symptoms after omega-3 fatty acid supplementation.
A study of Gustafsson et al. [9], investigated the efficacy of oral EPA supplementation (500 mg EPA; 2,7 mg DHA/day) in children with diagnosed ADHD to achieve relief of ADHD-symptoms. Partially significant results were recorded: There was a significant reduction in the symptoms inattention (p=0.04), cognitive impairment (p=0.04), oppositional behavior (p<0.03), hyperactivity (p<0.03) and impulsivity (p<0.03). The study concludes, that children with low EPA blood levels show a bigger benefit from EPA supplementation than children with higher EPA concentrations in the blood (p=0.02). A lack of omega-3 fatty acids, like EPA, might be caused by inadequate dietary intake, accelerated omega-3 fatty acid catabolism or a dysfunction in the synthesis of consumed essential fatty acids to EPA and DHA in the body [5].
The trial of Milte et al. [8], showed a relation between increased blood levels of EPA and DHA and the reduction in ADHD- symptoms after the treatment. The study analyzed the effects of oral EPA and DHA supplementation in children with diagnosed ADHD or ADHD-symptoms without diagnosis. Results included reduced hyperactivity (p<0.001), lower cognitive impairment (p<0.001), increased spelling ability (p<0.003) and an improvement in showing attention (p<0.001). However, significant symptom reduction was only achieved in children, who showed higher blood levels of EPA and DHA after the treatment [8].
Another study of Bélanger et al. [10], investigated the same issue like Milte et al. [8], however, with a more precise dosage of EPA and DHA supplementation measured per kilogram of body weight (20-25 mg EPA; 8.5-10.5 mg DHA/kg body weight/day). Next to significant symptom reduction, almost 90% of the children´s guardians perceived omega-3 fatty acid supplementation as a sensible alternative to drug therapy and supported this intervention [10].
Sinn et al. [11], investigated the possibility of optimizing ADHD- typical behavior in children without ADHD diagnosis by oral supplementation of EPA, DHA, certain micronutrients and the omega-6 fatty acid γ-linolenic acid and achieved impressive, significant results: The symptoms inattention, hyperactivity, impulsivity, oppositional behavior, restlessness, cognitive impairment as well as social problems were affected positively (p<0.01). They identified a correlation between PUFA deficiency in red blood cells and increased learning deficits and behavioral problems in children with ADHD symptoms. Thus, supplementation of PUFA might be beneficial for these children [11].
Contrary, a trial of Widenhorn-Müller et al. [7], which investigated the effects of EPA and DHA supplementation on behavioral changes in children with diagnosed ADHD and complementary comorbidities, only achieved a reduction of one symptom.
The interpretation of the above-mentioned studies shows a correlation between the disorder´s manifestation stage and the possible reduction of symptoms due to omega-3 fatty acid supplementation. A participant´s ADHD diagnosis was necessary for the study inclusion in the trials of Gustafsson et al. [9], Bélanger et al. [10] and Widenhorn-Müller et al. [7]. Sinn et al. [11], as well as Milte et al. [8], did not necessarily require an ADHD diagnosis for study participation. Investigations with exclusively ADHD- diagnosed patients achieved less significant behavioral changes of ADHD-symptoms compared to investigations without mandatory ADHD diagnosis. This indicates, that supplementation of omega-3 fatty acids may be an effective treatment option for mild forms of ADHD[18]. This assumption can be confirmed by further available investigations. Manor et al. [19], as well as Vaisman et al. [20], investigated omega-3 fatty acid supplementation exclusively in ADHD-diagnosed subjects and resulted minor behavioral improvement. Contrary, the study by Richardson et al. [16], investigates children with ADHD symptoms, but without medical diagnosis and showed significant improvement in learning deficits and behavior due to PUFA supplementation [18]. A detailed overview of the mentioned studies including the number of participants, intervention duration and used placebos as well as the exact dosages is given in Table 1.
| Authors | Population | Intervention | Comparator | Duration | Results significant relief from: |
|---|---|---|---|---|---|
| Milte et al. [8], (Australia) |
n=53 age: 6-13 years no ADHD diagnosis |
oral EPA and DHA supplementation in capsule form dosage/day*: 264 or 1109 mg EPA; 108 or 1032 mg DHA |
safflower oil | 12 months | oppositional behavior cognitive impairment literacy deficits inattention |
| Widenhorn- Müller et al. [7], (Germany) |
n=95 age: 6-12 years ADHD diagnosis |
oral EPA and DHA supplementation in capsule form dosage/day: 600 mg EPA; 120 mg DHA |
olive oil | 16 weeks | cognitive impairment |
| Gustafsson et al. [9], (Sweden) | n=92 age: 7-12 years ADHD diagnosis |
oral EPA supplementation in capsule form dosage/day: 500 mg EPA; 2, 7 mg DHA |
rape oil | 15 weeks | inattention cognitive impairment oppositional behavior hyperactivity/impulsivity |
| Bélanger et al. [10], (Canada) | n=26 age: 6-12 years ADHD diagnosis |
oral EPA and DHA supplementation in capsule form dosage/day: 20-25 mg EPA; 8, 5-10, 5 mg DHA/kg body weight |
sunflower oil | 16 weeks | inattention impulsivity |
| Sinn et al. [11], (Australia) | n=87 age: 7-12 years no ADHD diagnosis |
oral PUFA supplementation in capsule form dosage/day: 558 mg EPA; 174 mg DHA; +micronutrient supplementation |
palm oil | 30 weeks | inattention hyperactivity/impulsivity oppositional behavior restlessness cognitive impairment social problems |
Note: * dosage per phase and experimental group.
Phase 1: Group 1: 1109 mg EPA, 108 mg DHA; Group 2: 264 mg EPA, 1032 mg DHA; Group 3: Comparator;
Phase 2: Group 1: 264 mg EPA, 1032 mg DHA; Group 2: Comparator; Group 3: 1109 mg EPA, 108 mg DHA;
Phase 3: Group 1: Comparator; Group 2: 1109 mg EPA, 108 mg DHA; Group 3: 264 mg EPA, 1032 mg DHA.
Table 1: Trials used and results.
However, several limitations in the study designs may not be ignored. The heterogeneity of the patients, which is mainly caused by the presence of comorbidities and the imbalance of male and female participants, must be considered. Furthermore, the variability of the dosed fatty acids, the type of administered placebo as well as possible responders or non-responders concerning the supplementation of omega-3 fatty acids may influence the outcome [7,8-11].
Supportive dietary recommendations concerning omega-3 fatty acids
Alpha-Linolenic Acid (ALA), EPA and DHA are omega-3 fatty acids and belong to the group of Poly Unsaturated Fatty Acids (PUFA). Dietary recommendations suggest an intake of 0.3 to 0.4 g of omega-3 fatty acids for adults per day. This corresponds roughly to the consumption of two fish dishes (in total approx. 210 to 280 g) per week [21,22]. During pregnancy and nursing period, women should take an average of 0.2 g DHA daily. Several trials show a positive impact of the child’s cognitive and visual development, if pregnant women consume a sufficient amount of fish and oils rich in omega-3 fatty acids [23-26]. An amount of 0.1 g of omega-3 fatty acids is recommended for six-month-old infants up to toddlers with two years. After this period until adolescence, a daily intake of 0.25 g of this fatty acids is recommended [21,22]. Sources for ALA are flax- and chia seeds, but also in walnut-, hemp-, rapeseed- and soybean-oil. Fatty marine fish and certain freshwater fish, like trout, contain EPA and DHA [27]. Tables 2 and 3 show foods rich in EPA and DHA as well as their total content of these fatty acids. Table 4 shows the food´s recommended frequency of consumption [27,28].
| Food (100 g) | EPA (mg) | DHA (mg) | Total content (mg) |
|---|---|---|---|
| High-fat fish | |||
| Sardine | 747 | 1.337 | 2.084 |
| Herring | 740 | 1.170 | 1.910 |
| Salmon | 593 | 1.155 | 1.748 |
| Mackerel | 588 | 739 | 1.327 |
| Tuna | 223 | 593 | 816 |
| Low-fat fish | |||
| Trout | 424 | 600 | 1024 |
| Codfish | 104 | 250 | 354 |
| Haddock | 59 | 124 | 183 |
Table 2: Fish rich in omega-3 fatty acids.
| Food (100 g) | Total content of EPA and DHA (mg) |
|---|---|
| Vegetable oils | |
| Flaxseed oil | 52.800 |
| Walnut oil | 12.200 |
| Rape oil | 9.600 |
| Wheatgerm oil | 7.800 |
| Soya oil | 7.700 |
| Nuts | |
| Walnut | 7.830 |
| Pecan nut | 757 |
| almond | 260 |
| Cashew nut | 150 |
| hazelnut | 109 |
Table 3: Vegetarian food rich in omega-3 fatty acids.
| Food | Recommended intake |
|---|---|
| Fish | Twice a week (in total 210 to 280 g) One portion high-fat fish One portion low-fat fish |
| Vegetable oils | Approx. one tablespoon a day (in total 10 to 15 g) |
| Nuts | Approx. 30 g per day |
Table 4: Recommended intake of food rich in omega-3 fatty acids.
For reducing ADHD-symptoms a recommended dose of omega-3 fatty acid supplementation is still under investigation [10]. The effectiveness of omega-3 fatty acid supplementation varies enormously and sometimes symptom reduction is only shown in subgroups of ADHD affected patients [5,29-31]. It should also be noted that supplementation of omega-3 fatty acids may not provide maximum benefit if there is a shortage of further micro- or macronutrients. PUFA metabolism is based on nutrients such as vitamin C, B3, B6, zinc, magnesium. Children with ADHD sometimes also show zinc and iron deficiency, which may affect the success of omega-3 fatty acid supplementation negatively [11].
In summary, oral omega-3 fatty acid supplementation can achieve symptom reduction in children with ADHD symptoms. Due to the inconsistent dosage of omega-3 fatty acid supplementation in the mentioned studies, positive results of improvement only can be shown limited. According to the current state of research, the optimal dosage of omega-3 fatty acids for children with ADHD symptoms cannot be generalized. It depends on the individual´s needs as well as the extent of omega-3 fatty acid deficiency. Nevertheless, this supplementation provides a safe and low-sideeffect treatment option and might represent a safe component of a multidisciplinary therapy concept. It has to be considered, that combined supplementation of omega-3 fatty acids with further nutrients like omega-6 fatty acids may also result in symptom reduction and show better results than an exclusive omega-3 fatty acid supplementation. A dose-reduced psychostimulants medication in combination with oral omega-3 fatty acid supplementation might also represent a therapy concept associated with long-term freedom of ADHD symptoms in children. However, due to the fact, that Attention/Hyperactivity Disorder (ADHD) is a multifactorial triggered disorder, it needs a multidisciplinary intervention team, including caring guardians, doctors, psychologists, teachers, and nutritional experts like dieticians and further occupational groups to successfully achieve symptom relief.
Hauser R and Salvador C contributed to conception and design of the review and drafted the manuscript. Erler J and Michel M contributed to acquisition and interpretation of data. Kropshofer G and Crazzolara R critically revised the manuscript. Salvador C gave final approval.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hauser RT, Erler J, Kropshofer G, Crazzolara R, Michel M, Salvador C (2022) Omega-3 Fatty Acid Supplementation as Therapeutic Option for Children with ADHD-Symptoms. Pediatr Ther. 12:457.
Received: 30-Nov-2020, Manuscript No. PTCR-22-001-PreQC-22; Editor assigned: 07-Dec-2020, Pre QC No. PTCR-22-001-PreQC-22 (PQ); Reviewed: 21-Dec-2020, QC No. PTCR-22-001-PreQC-22; Revised: 30-May-2022, Manuscript No. PTCR-22-001-PreQC-22 (R); Published: 29-Jul-2022 , DOI: 10.35841/2161-0665.22.12.457
Copyright: © 2022 Hauser RT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.