Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Short Communication - (2024)Volume 15, Issue 2
Most undergraduate Organic chemistry textbooks contain a section on the general acid-base equilibria, strength of acids and bases, and predicting the equilibrium position of acid-base reactions (1-3). The students are taught to qualitatively predict the directions of these reactions using only the acid/base strengths or acid/base constants (pKa/pKb) without elaborating how or why they work. For example, statements like ‘stronger acids/bases tend to move towards weaker forms’ are not good explanations (1-3). Moreover, finding an answer in analytical and physical chemistry textbooks specific to this question is challenging (4-6). The simple thermodynamic framework put forward here can easily explain why these predictions work and allow one to quantify the direction of acid-base reactions.
For a general acid-base reaction, consider an acid HA transferring a proton to the conjugated base B¯ of an acid HB in an aqueous solution. The reaction forms the conjugated base (A¯) of the first acid, and HB.
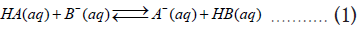
The thermodynamic equilibrium constant (Keq) for this reaction can be written as,
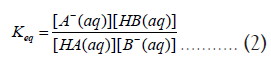
According to equation (2), if the reaction moves forward, i.e., to convert most of the reactants into products, the equilibrium constant should be greater than unity (Keq>1). Furthermore, the second law of thermodynamics states that a chemical process that moves forward should produce a negative Gibbs free energy change (ΔG0Overall) for the overall reaction (1-6) [1]. In addition, ΔG0Overall can be related to Keq by the following equation,
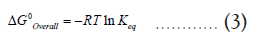
From equation (3), it is clear that a reaction with Keq>1 is also characterized by a negative (ΔG0Overall) value. The next question is how to relate these thermodynamic quantities to pKa/pKb values of acids and bases. To do that, consider individual reactions of HA and HB with water, where they transfer a proton to a water molecule acting as acids in an aqueous solution [2].
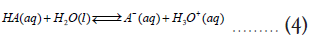
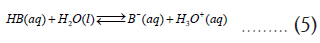
From these two reactions, we can define their acid constants (Ka) as follows,
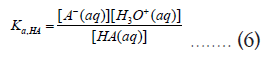
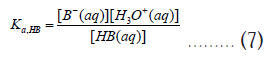
As before, these thermodynamic equilibrium constants can be related to corresponding Gibbs free energy changes as follows,
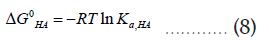
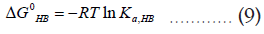
Comparing the chemical equation (1) with (4) and (5), it can be shown that,
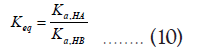
Then the equation (3) can be written as,

Converting the ln terms into a log terms and realizing pKa = − log Ka produces,
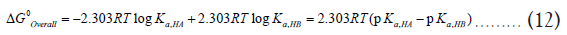
The equation (12) can be directly used to calculate the feasibility and extent of a reaction using the acid/base constants of individual components [3]. For example, if pKa,HA<pKa,HB, then the value of ΔG0Overall will be negative, and hence HA will transfer a proton to the B¯ to drive the reaction forward. The magnitude of ΔG0Overall determines the extent of the reaction. However, if ΔG0Overall is positive, the reaction is thermodynamically disfavored in the forward direction and favored in the reverse direction.
In addition, if the pKa of HA and pKb of the B¯ are given, one can modify the equation (12) as follows,
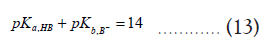
Then,
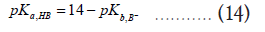
Plugging the equation (14) into equation (12) yields,
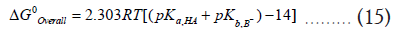
Equation (15) predicts that for the forward reaction to be thermodynamically feasible, the summation of the two pKa of HA and the pKb of the base B should be less than 14, or otherwise, the reverse reaction is more thermodynamically feasible [4].
Finally, we can demonstrate the applicability of the discussion in an actual example. If the pKa of Hydrocyanic acid (HCN) is 9.22 and that of acetic acid (CH3COOH) is 4.74, determine the favored determine for the following reaction [5].
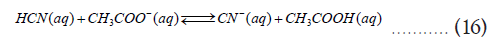
Let’s apply the equation (12) and calculate the ΔG0Overall for the above reaction assuming standard conditions (T=298.15 K and 1 atm pressure) and R=8.314 Jmol-1K-1
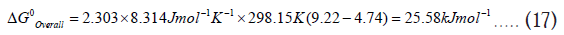
This calculation predicts that aqueous HCN will not transfer a proton to aqueous CH3COO¯ under standard conditions to drive the reaction forward [6]. However, acetic acid would readily transfer a proton to aqueous cyanide to drive the reverse reaction in a highly exergonic fashion.
The simple thermodynamic framework explained here should allow the students to understand the basis for using Ka/pKa values to predict the feasibility of acid-base reactions more logically and quantitatively and directly compare the robustness of different reactions. In addition, it may help them make connections between materials learned in Organic chemistry, such as the above example, and things they learn in analytical and physical chemistry on acid-base reactions, chemical equilibrium, and thermodynamics.
Citation: Senavirathne G (2024) On the Thermodynamic Feasibility of Acid-Base Equilibria. J Chem Eng Process Technol. 15:497.
Received: 06-Apr-2024, Manuscript No. JCEPT-24-30665; Editor assigned: 09-Apr-2024, Pre QC No. JCEPT-24-30665 (PQ); Reviewed: 24-Apr-2024, QC No. JCEPT-24-30665; Revised: 01-May-2024, Manuscript No. JCEPT-24-30665 (R); Published: 08-May-2024 , DOI: 10.35248/2157-7048.24.15.497
Copyright: © 2024 Senavirathne G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.