Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2020)Volume 11, Issue 4
Objective: To detect qualitative and quantitative analysis of the choroidal neovascular membrane based on opticalcoherence tomography angiography OCTA in response to treatment with anti-angiogenesis.
Patients and methods: This is a prospective observational study recruited 20 eyes of 20 patients with treatment-naïveCNVM. Patients were examined using the OCTA before and after treatment with Ranibizumab. Patients werefollowed-up monthly over 3 successive months and 1 month after the last injection. The collected data werestatistically analyzed using SPSS software version 22. Repeated measures ANOVA was performed and a paired t-testwas also used for comparison. Main outcome measures: membrane types, patterns, corrected visual acuity, retinalfluids, membrane size, and flow area; all were measured pre and post-intervention.
Results: Five patients were males and 15 were females (50.6 ± 17.7 years). Different configuration were detected; adensenet configuration in 6 eyes, a loosenet configuration in 6 eyes, mixed net in 3 eyes, and an unidentifiablepattern in 5 eyes. Visual acuity improved post-injection in all membrane patterns (p value 0.002 in loose-net andunidentifiable types). Signs of membrane activity, size, and flow area; all decreased in all patterns following treatment(p value 0.001).
Conclusions: OCTA in conjunction with OCT is both needed for more precise evaluation of differentmorphological and functional membrane criteria at which treatment strategy is based. Detection of blood flowchanges within the membrane itself can be considered as reliable biomarkers in monitoring the membrane activity.
CNVM; OCTA; OCT; Anti-VEGF; Flow area; CNV size; Activity signs
Choroidal neovascularization; CNVM is associated with abnormal vascular growth from the choroid. The neovascular lesion is classified as type 1 CNVM when expanding through Bruch’s membrane into the space beneath the retinal pigment epithelium (RPE) and type 2 when spreading into the subretinal space. Abnormal vessel proliferation may also originate from the retinal vasculature and is then referred to as retinal angiomatous proliferation or type 3 CNVM as described by Freund et al. [1].
Holz et al. described that fluorescein angiography (FA) was the gold standard for identifying the neovascular component. Optical coherence tomography (OCT) possibly in combination with FA and indocyanine green (ICGA) should be used to define macular neovascular disease. OCT has become an important non-invasive and quick method to diagnose and monitor the disease during anti-vascular endothelial growth factor (anti- VEGF) treatment by revealing sequelae of active CNV such as intra-retinal and subretinal fluid (SRF) accumulation and retinal thickening [2].
De Carlo et al. proved that Optical Coherence Tomography Angiography (OCTA) allows non-invasive visualization of retinal and choroidal vascular flow via motion contrast imaging [3]. Recent reports by Parravano et al. and Wang et al. described the potential clinical value of OCTA for monitoring treated CNV [4,5]. Schneider and Fowler reported that OCTA analysis of CNV morphology has shown the transition from an immature to mature phenotype following treatment with assessment of CNV type and identification of asymptomatic one [6]. Lumbroso et al. described that after each injection, a pruning of smaller vessels is seen immediately (after 24 hours) that increases for 6 to 12 days when it reaches a maximum [7]. It is followed by a reopening or new sprouting of the vessels 20 to 50 days later. This pruning leads to strengthening the trunk. In OCTA, closing terminal vessels (anti-VEGF effects) leads to an increased flow in the trunk after CNV reactivation. Anti-VEGF is considered to prune vascular sprouts of CNV resulting in increased flow through the remaining vessels leading to arteriogenesis with an increase in vascular diameter. Vascular sprouts regrowth may form connections between afferent and efferent vessels, and after numerous alterations, these connections enlarge to shunt vessels as described by Sulzbacher et al. [8].
This study aimed to detect OCTA role in monitoring activity of CNV along the course of treatment with Anti-VEGF.
This is a prospective observational study that took place from August 2017 to February 2018 at Ophthalmology Department, Fayoum University Hospitals, Fayoum Governorate in Upper Egypt. The study followed the declarations of Helsinki and was approved by the Fayoum University Hospital Ethics Committee.
The study included 20 eyes of 20 patients, presenting with choroidal neovascularization due to different etiologies as neovascular AMD, pathological myopia, multifocal choroiditis, and trauma. All of them were treatment-naïve. Patients with previous treatment by Anti-VEGF or photodynamic therapy were excluded.
All patients underwent a routine ophthalmic examination including best-corrected visual acuity (BCVA) testing using Snellen charts in Snellen ratios and Decimals and biomicroscopic examination. They were examined by OCTA using Angio-Vue (Optovue, Fremont, CA, USA). At the primary presentation, diagnosis of active CNV was made by the conventional FA. The neovascular activity was assessed using SD-OCT imaging by evidence of intra-retinal IRF and/or SRF.
OCTA images were evaluated using the SSADA algorithm to visualize retinal and choroidal vasculature in 3 × 3 mm and 6 × 6 mm of the central part of the retina. OCTA images were correlated with OCT B-scans, allowing visualization of both retinal flow and non-vascular structure.
Different CNV-vessel patterns based on OCTA according to Sulzbacher et al. were assessed [8]. The loose-net (LN) presented as individual vessels of large diameter, well-defined and discernible with low branching index showing no capillary sprouting. The dense-net (DN) appeared as a hyperreflective vascular net with dense capillary branching. Lesions with a ratio of approximately 50% of areas with large vessel diameter/low branching index and approximately 50% of areas with dense capillary branching were identified as the mixed type. Unidentifiable CNV pattern term was used when no neovascular vessels were detectable (neither in the choriocapillaris CC nor in the outer retina).
The CNV type and its site according to association with retinal layers were assessed according to Farecki et al. Type 1 CNV was a vascular tree presented in choriocapillaris slab not reaching the outer retina slab [9]. In contrast, Type 2 CNV demonstrated as a vascular tree originated at choriocapillaris slab and reaching outer retina slab.
Darwish classified AMD (according to OCTA lesions) into 2 patterns; a pattern I requiring treatment and pattern II not requiring treatment [10]. Pattern I showed all or at least three of the following five features; a well-defined CNV (tortuous lacywheel shaped), branching pattern (numerous tiny capillaries), presence of anastomoses and loops, morphology of the vessel terminals (presence of a peripheral arcade) and perilesional hypo-intense halo. Coscas et al. provided these criteria as a basis for analysis and evaluation of CNV activity and the degree of CNV proliferation, persistence and/or recurrence; conversely, they provide for the stabilization and healing with vessels that become mature or quiescent [11]. While a CNV lesion was considered as pattern II if it showed less than three of the previously reported OCTA features.
We measured the CNV size of the neovascular tree using a manual caliper. The CNV flow area was also assessed. Signs of activity were proved if it showed all or at least three of the previously mentioned five features of pattern I.
Patients were treated with three successive intravitreal injections of Anti-VEGF agent (Ranibizumab) with monthly intervals. Prior to injection, the pupil was dilated with tropicamide. Under topical anesthesia (Benoxinate eye drops) and complete aseptic precautions, the patient was asked to look away from the injection site, which was marked at a point 3.5 mm (if aphakic or pseudophakic) or 4 mm (if phakic) posterior to the limbus avoiding the horizontal meridian. The injection needle was inserted aiming towards the center of the globe, slowly delivering the injection volume (0.5 mg in 0.05 ml Ranibizumab) then the needle was removed slowly. A different scleral site was used for subsequent injections so that the same site was not injected repeatedly. Topical moxifloxacin q.i.d was given for 5 days postoperatively.
All patients were followed up with OCT and OCTA two weeks after each injection and one month after the third injection. Signs of activity, CNV size, flow area, and BCDVA were looked for at each visit.
The collected data were organized, tabulated and statistically analyzed using IBM SPSS software statistical computer package windows version 22 (2013, Armonk, NY, USA. IBM Corporation). Quantitative data were statistically described in terms of mean ± standard deviation (± SD). Repeated measures analysis of variance; ANOVA was performed to compare between means of BCVA at different times. Paired t-test was used to compare between means of CNV size and flow area pre and post-intervention. Qualitative data were described as frequencies (number of cases) and percentages. For interpretation of results of tests of significance, significance was adopted at P ≤ 0.05.
Twenty eyes of 20 patients presenting with CNVM due to different etiologies were included in the study. Five patients were males and 15 were females with a mean age of 50.6 ± 17.7 years.
Regarding CNV-vessel morphology based on OCTA, a DN pattern was present in 6 patients, whereas a loose-net (LN) pattern was present in 6 patients. Three patients showed a mixed pattern and an unidentifiable pattern was present in 5 patients.
The mean pre-interventional BCVA was 0.07 ± 0.06 decimal (6/95-6/75 Snellen). BCVA was variable pre and post-injection according to the pattern of CNV as shown in Figure 1.
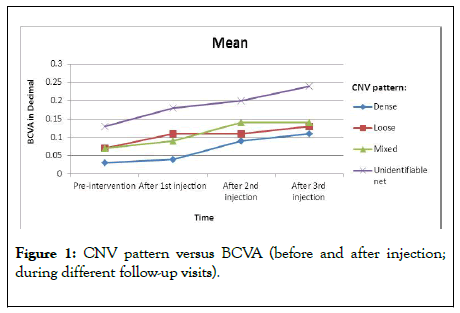
Figure 1: CNV pattern versus BCVA (before and after injection; during different follow-up visits).
The patients presented with unidentifiable net associated with the highest median BCVA compared to other types (0.13 ± 0.07) which improved gradually to 0.18 ± 0.08, 0.20 ± 0.11, and 0.24 ± 0.11 after the first injection, second injection and third injection respectively. There was a gradual improvement in BCVA in all patients with different patterns after repeated injections as shown as an example in Figure 2 with a significant difference in LN and unidentifiable types (p value 0.002).
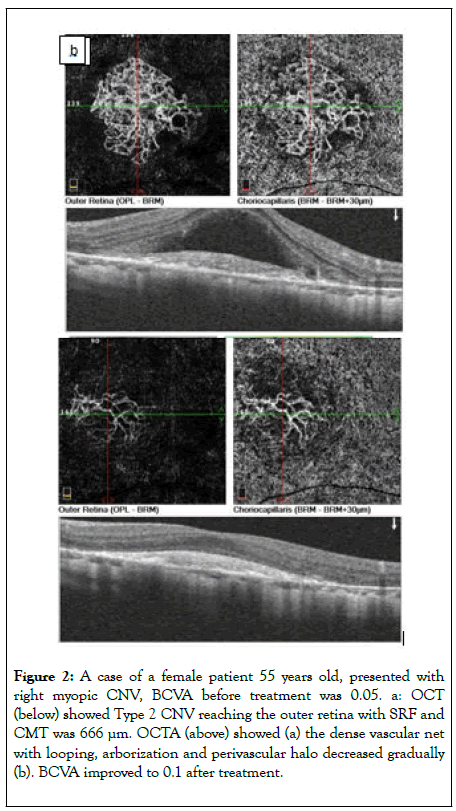
Figure 2: A case of a female patient 55 years old, presented with right myopic CNV, BCVA before treatment was 0.05. a: OCT (below) showed Type 2 CNV reaching the outer retina with SRF and CMT was 666 μm. OCTA (above) showed (a) the dense vascular net with looping, arborization and perivascular halo decreased gradually (b). BCVA improved to 0.1 after treatment.
In correlation to the type; two patients had CNV type 1 with median BCVA 0.17 before treatment which improved gradually after injection to become 0.2, 0.2 and 0.25 after first, second and third injection respectively as shown in Figure 3. Mean BCVA at baseline was 0.06 in 18 patients having CNV type 2, which improved significantly during the follow up period (p<0.0001); (Figure 4).
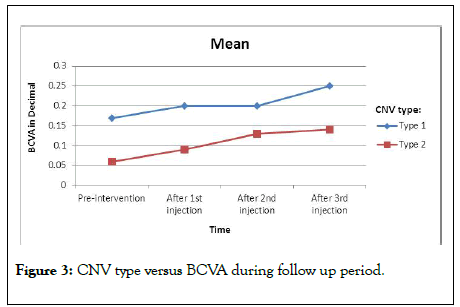
Figure 3: CNV type versus BCVA during follow up period.
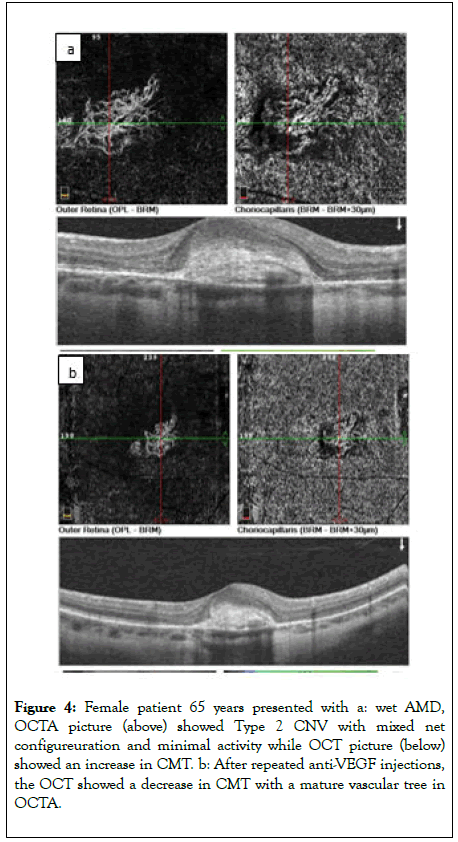
Figure 4: Female patient 65 years presented with a: wet AMD, OCTA picture (above) showed Type 2 CNV with mixed net configureuration and minimal activity while OCT picture (below) showed an increase in CMT. b: After repeated anti-VEGF injections, the OCT showed a decrease in CMT with a mature vascular tree in OCTA.
The activity of CNV had been evaluated according to changes in the structural OCT during the course of treatment by the presence of cystoid macular edema (CME), SRF, fibrovascular pigment epithelial detachment (FPED) and macular thickening. There was a gradual decrease in CMT along the treatment course with repeated injections. The mean CMT prior to injection was 290 ± 102.75 Mm that regressed gradually to be 229.15 ± 31.58 Mm after the 3rd injection. CMT was a good prognostic factor for the improvement of BCVA after repeated injections. The lower CMT, the better the improvement in BCVA. 50% of our patients (10 eyes) had CME at baseline which gradually decreased with repeated injections and disappeared completely after the third dose. Patients presented with dense-net type had SRF in 42.9% and IRF in 30% of cases by SD-OCT with different presentation in other patterns as shown in Figure 5.
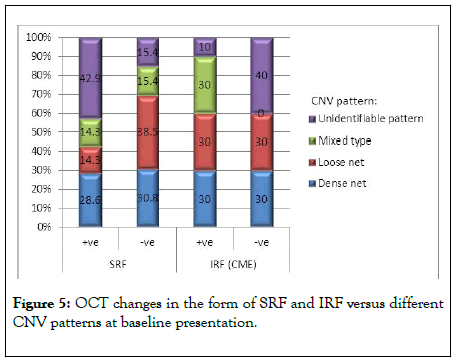
Figure 5: OCT changes in the form of SRF and IRF versus different CNV patterns at baseline presentation.
There was a gradual decrease in CMT along the treatment course with repeated injections. The mean CMT prior to injection was 290 ± 102.75 Mm that regressed gradually to be 229.15 ± 31.58 Mm after the 3rd injection. CMT was a good prognostic factor for the improvement of BCVA after repeated injections. The lower CMT, the better the improvement in BCVA. Fifty% of our patients (10 eyes) had CME at baseline which gradually decreased with repeated injections and disappeared completely after the third dose. Patients presented with dense net type had SRF in 42.9% and IRF in 30% of cases by SD-OCT with different presentation in other patterns as shown in Figure 5.
Seven patients (35%) had SRF at baseline (range 448-458 μ) which gradually decreased during the follow up period and disappeared completely after the third injection. SRF was considered a positive marker for therapeutic response to anti- VEGF treatment, but a poor prognostic factor to improvement in BCVA after injection. Only two patients had PED before treatment, which gradually decreased along the course of treatment and disappeared completely at the end of follow up.
After three doses of anti-VEGF treatment, no activity was detected by OCT neither SRF nor IRF and by OCTA; all patterns became unidentifiable. In 14 eyes, the signs of activity by OCTA as vascular anastomosis, arborization, and perivascular halo, were associated with SRF, IRC and retinal thickening by structural OCT. Three eyes were presented by an unidentifiable net, in which the signs of activity by OCTA were inconclusive, while associated with SRF, IRC or retinal thickening by structural OCT. Although three eyes were presented with a dense capillary net with arborization, anastomosis and perivascular halo by OCTA but they didn’t associate with signs of activity by OCT.
We used OCTA for precise quantitative analysis of CNV before treatment and evaluated the changes after treatment, this assessment included measurement of CNV size and flow area. In our study, the mean CNV size was 1.64 ± 1.06 mm before treatment which decreased gradually post-injections to 1.17 ± 0.73 mm (p value 0.001). We also evaluated the CNV flow area which was 1.29 ± 0.71 mm before treatment that decreased gradually after 3rd injection to 0.89 ± 0.52 mm (p value 0.001). Both CNV size and flow area decreased which indicated effective and good response to treatment as shown in Figure 6.
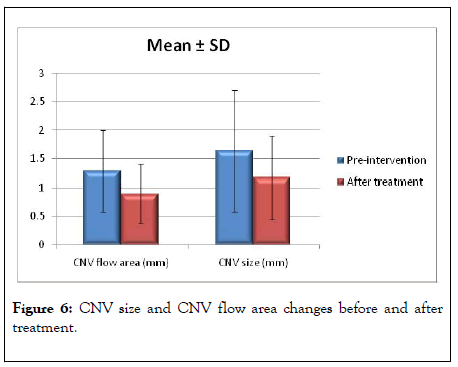
Figure 6: CNV size and CNV flow area changes before and after treatment.
In this study, 18 eyes showed gradual regression in response to Anti-VEGF along the course of injection (three times). Only two eyes showed initial regression in activity after the first injection and became stationary after the second and third injections.
Choroidal neovascularization CNV as described by Gehrs et al. is frequently accompanied by various ophthalmic diseases in the posterior segment and is a key to pathologic change, causing severe vision loss [12]. Our study aimed to identify characteristic CNV vascular patterns using OCTA imaging and follow up the activity during treatment with anti-VEGF repeated injections and detect the response to treatment. We recruited 20 eyes with treatment-naïve active CNV.
In our study, we evaluated the morphological changes of CNV and transition from active lesion to an inactive lesion in response to treatment. We evaluated CNV activity by OCTA according to criteria described before by Darwish and Coscas et al. [10,11] Eighteen eyes became inactive after three successive doses of anti-VEGF with only two eyes became inactive after one dose of injection. When neovascular lesion became inactive according to the criteria discussed before, it meant that this vascular tree became mature with large trunk diameter and low branching of peripheral arcades.
FA provides dynamic information currently not available with OCT angiography such as transit time of fluorescein dye to travel to the eye. Additionally, Jia et al. described that changes in patterns of fluorescence over time are used for identifying various pathological conditions as well as the assessment of disease activity, such as CNV. Similarly to Jia et al. , we used FFA as an initial step for diagnosis of CNV to detect its site, type, and cause [13]. However, the study depended on OCTA in monitoring CNV morphological changes in response to treatment as it is a non-invasive, rapid method and dye-free.
Sulzbacher et al. used OCTA for morphological classification of treatment-naïve CNV and following repeated injections. In our study we used their classification for morphological analysis of our 20 patients [8]. Miere et al. reported that the mean CNV area at baseline was 1.58 ± 1.72 mm with a decrease in CNV total area of 21.6% [14]. CNV size and flow area decreased in all 17 eyes after anti-VEGF treatment. However, by qualitative analysis after injections, CNV lesions were classified into 2 patterns; constant patterns which conserve its morphology with tiny capillaries and loops were suggestive for immature CNV, changing patterns with arterialization with thicker dilated vascular trunks and absence of tiny arborization, suggestive of mature neovascular lesions. We agreed with Miere and cowrokers in this quantitative analysis, as all our patients showed a gradual decrease of CNV flow area after the 3rd injection to 0.89 ± 0.52 mm.
Our study coincided with that of Schneider and Fowler, all patients changed from immature to mature CNV pattern in response to treatment [6].
Spaide et al. described that active DN in treatment-naive lesions was often associated with leakage into all retinal compartments (IRF, SRF, FPED), mostly SRF, a positive marker for a therapeutic response during the early intervention. We agreed with Spaide et al. , as 35% of our patients had SRF at baseline. 42.9% of them had DN configureuration. In all of those patients, SRF disappeared completely after repeated injections [15].
OCTA can differentiate various types of CNV in exudative AMD. Similarly to Farecki et al. , we evaluated patients with CNV due to different etiologies and were classified by OCTA into type 1 (only two patients) and 18 patients had type 2 [9].
Morphologic and functionally relevant sequelae for CNV activity are numerous as described by Tam et al. [16]. In our study, we evaluated CNV activity by OCT according to CME, SRF, PED and retinal thickening. 50% of patients had CME before treatment which disappeared after treatment; as they responded well to injections but with low BCVA. 35% of patients had SRF at baseline which disappeared after treatment as they also had a good response to anti-VEGF but with poor improvement in BCVA. Only two patients had PED before treatment which disappeared after treatment. Three eyes had no changes in structural OCT but there was an active vascular tree confirmed by OCTA, so OCT alone was not a conclusive tool for evaluation of CNV activity.
Spaide et al. described that advanced image analyses indicated a strong impact of fluid localization within different compartments of the retina [15]. Intraretinal cysts (IRC) were consistently associated with the lowest BCVA, whereas SRF demonstrated superior BCVA. In our study, we observed that patients had IRC or SRF presented with the lowest BCVA which proved to be poor prognostic factors for visual improvement in response to treatment.
Sulzbacher et al. stated that OCTA visualizes the pathologic correlate of neovascular AMD with distinct pattern characteristics about lesion location at different layer depth [8]. Our study revealed dense neovascular nets as a candidate for early disease activity and LN as a probable marker for advanced scarring or vascular maturation.
Iacono et al. described that Ranibizumab therapy was effective in improving and maintaining visual acuity in myopic CNV [17]. Early diagnosis and better baseline BCVA were strongly associated with better functional outcomes. CNV distinguished by its small size and low CMT responded more favorably, achieving a better visual outcome. We agreed with Iacono et al. , as patients with low CMT had better BCVA at baseline and they responded well to anti-VEGF treatment with better visual outcomes.
In our study, 18 eyes showed gradual regression in response to Anti-VEGF along the course of injection (three times). Only two eyes showed regression in activity after the first injection and became stationary after the second and third injection.
In 14 eyes, the signs of activity by OCTA as vascular anastomosis, arborization, and perivascular halo, were associated with SRF, IRC or retinal thickening by structural OCT. Three eyes were presented by an unidentifiable net, in which the signs of activity by OCTA were inconclusive, while associated with SRF, IRC or retinal thickening by structural OCT. Although three eyes showed signs of activity by OCTA, they were not associated with signs of activity by OCT. So both OCT and OCTA were complementary to each other in the evaluation of disease activity and detection of response to treatment.
Unfortunately, we couldn ’ t evaluate the changes in PED at different times as it was only detected in 2 patients before treatment. We have missed the absence of ELM visibility in myopic cases as a sign of activity as reported by Battaglia et al. , this is considered a limitation [18]. However, we evaluated different morphological changes of CNVM during the follow-up period after treatment with anti-VEGF using OCTA which was discussed in a few publications as far as we know.
Our study demonstrated that all patients need FA as an initial step in the diagnosis, to detect CNV site and cause. But, OCT and OCTA are the gold standard methods for more precise evaluation of CNV type, pattern and different morphological and functional criteria at which treatment strategy is based. So both types should be used in conjunction with each other and none of them is conclusive alone. Changes in the membrane area and blood flow within the membrane itself can be considered as reliable biomarkers in monitoring the membrane activity.
Citation: Sayed SSE, Kamal MA, Sadek SH, Hatata RM (2020) Optical Coherence Tomography Angiography Evaluation of Morphological andVascular Changes in Choroidal Neovascularization in Response to Anti-Angiogenic Treatment. J Clin Exp Ophthalmol. 11:847. DOI:10.35248/2155-9570.20.11.847
Received: 28-May-2020 Accepted: 11-Jun-2020 Published: 18-Jun-2020 , DOI: 10.35248/2155-9570.20.11.847
Copyright: © 2020 Sayed SSE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, whichpermits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.