Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Research Article - (2014) Volume 5, Issue 1
Entrance Surface Dose (ESD) is one of the basic dosimetric quantities for measuring the patient dose and hence, an excellent tool for optimization purposes and for comparison with the international reference values. ESD value measurement for patient is also, an essential component of the quality assurance program for individual X-ray radiology departments. Factors affecting dose in all imaging modalities include beam energy, filtration, collimation, patient size, and image processing. Organ absorbed dose can be estimated by using a conversion factor along with a measured value of entrance exposure. When estimating the radiation dose of an individual patient, patient specific calculation methods can be used. The main objective of this study was to develop methods for assessment of ESD. In this study, image quality is quantified by modeling the whole X-ray imaging system, including the X-ray tube, and patient. This is accomplished by using Monte Carlo (MC) simulation methods that allow simultaneous estimates of measures of image quality and patient dose. In This study MCNP4C code was used to state a model for both human body and X-ray machine, to carry out such an investigation. Mathematical model of the human body with its all internal organs was used, and an image receptor of variable thickness and composition. Experimental results showed good agreement with theoretical predictions. The model may be used to generate data for a range of exposure conditions, and sample results will be presented. The usefulness and limitations of such a theoretical model will be discussed.
Keywords: Monte Carlo method; Diagnostic X-ray; ESD
X-ray diagnostic machines-one of the most widely used man made radiation sources- are now part of any patient’s life, after, before and also sometimes during treatment for any problem. There are fundamentally two reasons for measuring or estimating radiation doses to patients; firstly measurements provide a mean for setting and checking standards of good practice as an aid to the optimization patient protection. Secondly, estimates of the absorbed dose to tissues and organs in the patients are needed to determine the risks so that diagnostic technique can be properly justified and cases of accidental overexposure thoroughly investigated [1].
Information that can be utilized to give a patient an accurate diagnosis, and subsequently a successful treatment is essential. However, imaging with ionizing radiation is also associated with a small risk for cancer induction or genetic detriment. When X-ray photons are scattered or absorbed in the cells of the human body, ionizations occur that can alter molecular structures and thus do harm to the cell. The most important damage to the cell is damaged in the DNA since this may induce mutations. Ultimately, the damage may lead to that the cell is killed, and if enough cells are killed, the function of the tissue or organ will be deteriorated. This type of acute harm due to large radiation exposures is referred to as a deterministic effect [2]. However, at the relatively low radiation exposures in diagnostic radiology, the damages caused by ionizing radiation are often rather easily repaired. Yet, sometimes the damage on the DNA is more complex. This can cause mutations or chromosomal aberrations, which in turn may lead to a modified cell but with a retained reproduction capacity. In some cases, such modified cells can result in a cancer. In the case where the harmful effects of ionizing radiation are only known, statistically, it is referred as a stochastic effect. The risk related to stochastic effects to a human from exposure from ionizing radiation is often quantified with the effective dose, E [3]. The intensity and quality of the radiation emerging from an X-ray tube are primarily a function of the tube current, exposure time, applied and filtration source quantities as identified in Figure 1. The most common method is the determination of the entrance surface dose using thermoluminescent dosimeters or calculating from the output of the X-ray unit and dose-area product. To insure the ESD without using these factors, Monte Carlo techniques for dose estimation to organs have been developed [4].
In this study, MCNP4C is used to simulate the diagnostic radiology X-ray tube with the aim of the predicting the X-ray spectra using various tube voltages (between 50 and 120 kV). The method, based on Monte Carlo technique, is integrated into flexible software capabilities to estimate the absorbed dose when the possibility of application of other actual methods does not exist.
Monte carlo codes
MCNP is a well-known general purpose Monte Carlo code for the transport of neutrons, photons and electrons developed at the Los Alamos National Laboratory. The user can apply up to second order surfaces (boxes, ellipsoids, cones, etc.) and fourth order tori to build a three-dimensional (3D) geometry, which can be filled with materials of arbitrary composition and density. Point, surface or volume sources of radiation can be defined, from which the mentioned particles are emitted with user specified probability distributions for energy and direction. The code then simulates the particle tracks and interactions with the materials, according to probability density distributions implied by particle and material properties. Taking a comprehensive account of the underlying physics of radiation-matter interaction, it creates secondary particles (which are also transported) and keeps a record of quantities like particle fluence, energy deposition and dose. The user indicates at what points, surfaces or volumes, these quantities are reported. For this paper MCNP version 4C was used, which has been implemented on a Compaq XP900 Alpha workstation. Without attempting optimization, i.e. the application of additional variance reduction techniques, it typically takes some 6 h of computer processing time (20 million starting particles) to yield less than 0.5% relative statistical uncertainty in the calculated effective dose conversion factor for patients [5].
Monte Carlo calculations simulate and record the energy deposition of X-ray photons in mathematically described anthropomorphic phantoms. The radiation interaction histories of a large number of incident photons are followed using known physical descriptions of the interaction processes and the resulting energy depositions at the sites of interaction are recorded [6]. For diagnostic radiology dosimetry the physical process treated are limited to the photoelectric effect and Compton scattering since the initial photon energies in the range of the intersect are less than 150 KeV [7]. The energy given to the secondary electrons is assumed to be absorbed at the same point, that is, the kerma is equal to the absorbed dose. The ranges of the secondary electrons are small compared with the dimensions of the study organs, and the absorbed dose does not change abruptly with distance except at the boundary where composition and density change [8]. These boundary effects have little impact in the determination of the average absorbed dose in the tissues. The one exception is the active bone marrow, where a small increase in absorbed dose due to the size of the marrow cavities is expected from increased photoelectron emission by surrounding bone [7,8].
Tissue doses are obtained by summing, in each organ, all energy depositions from primary and scattered photons, and dividing by the total organ mass. The result is the average absorbed dose in the entire organ regardless of the fraction of the organ.
The body is represented as erect with the positive z-axis directed upward toward the head. The x-axis is directed to the phantom’s left, and the y-axis is directed toward the posterior side of the phantom. The origin is taken at the center of the base of the trunk section of the phantom [7].
This study presents a model for the human body that was done using MC technique. In Figure 2(a) the model as given by the output of the used code is shown which was stated on that given in (b) [9], some of the organs such as spine, kidneys and the rest of the respiratory system do not appear according to the drawing section. The elemental compositions for all human organs and for the adipose tissue were derived from data in ICRP 89 [10]. As composition and tissue density are important parameters in determining the transport of photons in the body, geometric shape of each organ in the human body is very essential in preparing the input of the code concerning with the relation between all these organs and they must not intersect. Cristy [11], gave these mathematical representations for different ages.
Trunk: The trunk, exclusive of the female breasts, is represented by a solid elliptical cylinder specified by:
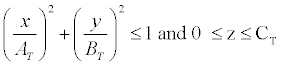 , where AT=17.25, BT=9.80 and CT=63.10
, where AT=17.25, BT=9.80 and CT=63.10
The trunk section includes the arms and the pelvic region to the crotch. The female breasts are appended to the outside of the trunk section.
Skull: The skull comprises the cranium and the facial skeleton. The cranium is represented by the volume between two concentric ellipsoids defined by:
where d=0.76 and the values a, b, and c are the same as the values a, b, and c given in the statements and table for the brain. The facial skeleton is represented by a portion of the volume between two concentric elliptical cylinders. The portion of the volume that intersects the cranium and brain is excluded. The inequalities are:
y ≤ 0, CT + z1 ≤ z ≤ CT + z5,
The variables a2, b2, and c2 correspond in numerical values with the variable expressions (a+b), (b+d), and (c+d), respectively, in the statements defining the cranium and hence are not given below.
a1=6.92, b1=8.1, d=1.10, z1=3.79, z5=14.05
Pelvis: The pelvis is a portion of the volume between two nonconcentric elliptical cylinders. The inequalities defining the pelvis are
y ≥ y02, 0 ≤ z ≤ z2, and y ≤ y1 if z ≤ z1, where a1=9.75, b1=11.07, a2=10.35, b2=11.76, Y01=-3.72, y02=-2.94, y1=4.90, z1=12.62, z2=19.83.
Spine: The spine is an elliptical cylinder given by
It is divided into 3 portions—an upper, middle, and lower—such that dose and absorbed fractions can be estimated separately for each portion. These divisions are formed by the planes z=z2 and z=z3.
A=1.73, b=2.45, y0=5.39, Z1=19.83, Z2=31.64, Z3=63.10, Z4=72.91
Entrance surface dose
Two types of dosimeter are commonly used for estimating ESD to patients during X-ray examinations, namely Thermo-Luminescent Dosimeters (TLDs) and ionization chambers. TLDs have the advantage of being physically small, enabling them to be stuck directly and unobtrusively on the patient’s skin with very little interference in patient mobility or comfort. They will fully measure the radiation backscattered from the patient, an essential component of the Entrance Surface Dose (ESD) and are unlikely to obscure useful diagnostic information. Ionization chambers, being more bulky and requiring connecting cables are usually difficult to attach in sufficiently close contact to the patient’s skin to ensure complete measurement of the backscattered radiation, severely restrict patient mobility and cast interfering shadows on radiographs [12]. They are consequently not recommended for direct measurement of ESD. They can, however, be used to make measurements of the absorbed dose to air, in free air, on the axis of the X-ray beam without a patient or phantom present. Such measurements can be corrected using appropriate backscatter factors and the inverse square law to estimate the ESD. In previous study, Victoreen 4000M+ was used to evaluate the dose to patients during different diagnosis as part of implement QC program in diagnostic radiology [13]. TLDs are recommended for direct measurement of ESD and are available in a variety of physical forms and in different materials. The National Radiological Protection Board (NRPB) recommends individual chips or pellets of lithium fluoride or lithium borate [12]. TLDs dosimeters (Harshaw TLD100) is undertaken in this study for validation of ESD with the calculation using by MCNP4C. The TLDs were read using a Harshaw 4500 TLD reader. The TLD energy response was 15% across the range 20–200 kVp, the uncertainty of measurement was estimated to be less than 10%. ESD is absorbed dose in soft tissue that would be measured at the point where the central axis of the x-ray beam enters the body. The five most frequently performed diagnostic radio-graphic examinations were included in the study; Skull, Chest, Abdomen, Pelvis and Lumper Spin. For each radiographic projection the mean patient weight was within the range of 70 kg. For each radiograph the tube potential, mAs, FSD, FFD, cassette size, patient weight and age were recorded. The image quality of all X-ray examinations included in the sample was satisfactory according to the radiologists of the department and fulfilled all image criteria set according to the European guidelines [14]. Radiographic condition used in definition of normalized organ dose is illustrated in Table 1 and Figure 3.
| Organ | Exposure parameters | ||
|---|---|---|---|
| kVp | mA | Sec | |
| Chest (PA) | 50-60 | 100 | 0.7 |
| Skull | 50-60 | 40 | 1 |
| Abdomen (AP) | 60-70 | 40 | 2 |
| Pelvis (AP) | 70-80 | 40 | 2 |
| Lumbar spine (AP) | 60-80 | 60 | 2 |
Table 1: Radiographic condition used in definition of normalized organ dose.
The contribution of backscattered radiation is to be included. ESD is related to the incident absorbed dose by the backscatter factor BSF thus,
ESD=(ID) (BSF)
BSF depends on the X-ray spectrum, the X-ray field size, the thickness of the patient and the distance between the center of the dosimeter and the surface [15]. The influence of this factor can be minimized by using a dosimeter of small volume directly attached to the patient’s skin or by recessing the dosimeter in the surface of the phantom [16].
Calculation of ESD from tube output
ESD=nKa (U, F) (100 cm/FSD)2 Pit BSF
Where
nKa (U, F) is the tube output (mGy/mAs) at a distance of 100 cm from the focus with high voltage U and total filtration F.
FSD is the focus to skin distance cm.
Pit tube current-time product used mAs.
Table 2 presents the mean values and standard deviation of measured and, as well as the calculated ESD for Skull, Chest, Abdomen, Pelvis and Lumper Spin examinations. As shown in Table 2, the mean ESD ranged from approximately 0.23 to 0.57 mGy for chest, from 2.03 to 5.1 mGy for skull, from 2.58 to 3.54 mGy for abdomen, from 6.3 to 13.6 for pelvis and from 4.1 to7.1 to lumber spine. The ESD measured by TLD are slightly higher than ESD in all. The main source of the high values of the experimental results may come from many factors such as: the reproducibility of exposure (within 2% for one standard deviation), variations due to the experiment geometry, and variations of TLD sensitivities (within 10%, for one standard deviation) [16]. It must be stressed that the TLD threshold dose not only depends on the annealing and measurement protocols used and the equipment available, but also on the particular batch of TLDs used for the ESD measurements. Therefore, more investigation should be done using other types of TLDs such as calcium Fluoride (CaF) dosimeters that are much more sensitive than LiF TLDs.
| Organ | kVp | Measured ESD | Error% | Calculated ESD | Error% |
|---|---|---|---|---|---|
| Chest (PA) | 50-60 | 0.23-0.57 | 0.01-0.05 | 0.19-0.6 | 23-5.3 |
| Skull | 50-60 | 2.03-5.1 | 0.03-0.08 | 1.7-5.5 | 16.3-7.8 |
| Abdomen (AP) | 60-70 | 2.58-3.54 | 0.05-0.09 | 2.34-3.84 | 10.9-8.5 |
| Pelvis (AP) | 70-80 | 6.3-13.6 | 0.07-0.1 | 6.12-13.97 | 2.9-2.7 |
| Lumbar spine (AP) | 60-80 | 4.1-7.1 | 0.1-0.2 | 3.8-7.4 | 7.3-4.2 |
Table 2: ESD (mGy) measured and calculated for different examinations.
The correlation between ESD calculated and ESD measured by TLD in all radiographic procedures included in the study were: PA chest: 0.89%; AP abdomen: 0.96%; AP pelvis: 0.97%; AP lumber spine: 1.03% and skull: 1.05%. Thus for all examinations studied, the correlation between calculated and measured doses was very high as shown in Table 2, Figures 4 and 5.
According to the European Commission (EC) and National Radiation Protection Board (NRPB), the mean ESD in all radiographic examinations being substantially lower than Guidelines dose reference levels (EC and NRPB Dose Reference Level). More attention should be taken for chest examinations, where, the mean ESD is two times higher than the DRL proposed by EC and DRLs recently proposed by NRPB [14,17] given in the Table 3 [17-20]. The practical and calculated data for each examination are compared with the data reported from different similar studies for each examination and presented in Figure 6.
| Organ | Egypt | UK [18] | DRL [17] | DRLEC [14] | Gracee [1] | Romania [1] | Slovinia [1] | Italy [19] | Portogal [1] | Serbia [20] |
|---|---|---|---|---|---|---|---|---|---|---|
| Chest (PA) | 0. 35 | 0.16 | 0.2 | 0.3 | 0.69 | 1.7 | 0.23 | 0.57 | 0.31 | 0.4 |
| Skull | 3.8 | 3.9 | -- | -- | 3.5 | 11 | -- | 7.38 | 7.27 | 1.15 |
| Abdomen (AP) | 3.1 | 6.0 | 6.0 | -- | ---- | --- | --- | --- | ---- | ----- |
| Pelvis (AP) | 9.8 | 4.4 | - | 10 | 12.5 | 13.2 | 3.8 | 7.77 | ---- | 2.0 |
| Lumbar spine (AP) | 5.8 | 6.1 | - | 10 | 18.9 | 17.6 | 6.9 | 8.9 | 5.95 | 4.4 |
Table 3: Comparison of mean values of ESD (mGy) in this study (Egypt) by several radiographic procedures surveyed in different countries.
From the data obtained in this study, radiodiagnostic facilities are required to implement a system for patient dose reviews based on comparisons with International, national and local Diagnostic Reference Levels (DRLs). This DRL can be verified through the Quality Assurance (QA) programme. The QA is important to assess the performance characteristics of X-ray, ensure optimal image quality and perform accure diagnosis.
If a wide variety of clinical situations or exposure conditions need to be simulated, a mathematical representation of the patient and theoretical calculation of the mean organ doses may be more suitable. Theoretical calculations of organ doses passage of X-ray photons through the phantom. The parameter defining the X-ray beam can be easily modified so that the organ doses relative to exposure or surface absorbed dose can be calculated for any desired diagnostic X-ray field.
The present work aimed to present this simulation of human body depending on the description of the reference man given by ICRP 23 [3] with material composition taking from ICRP 89 [21] using Monte Carlo code MCNP4c. So the obtained results gave a useful tool for calculate ESD in diagnostic X-ray for different KVp and for any organ in the human body in few second which is an important way to estimate the dose received by the patient in every exposure.
Thus, Monte Carlo techniques dealt most of all the instrumental problems and provide reproducible results for any combination of the beam quality and the field size. Any range of Tissue-Air Ratios (TAR) values can be tabulated when the theoretical X-ray spectrum matches to the output spectrum of X-ray system used in practical studies.