Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2024)Volume 15, Issue 2
Moving towards personalized medicine brings the novel challenge of connecting highly specific treatments with the patients who can benefit from them. One such treatment is the cell therapy Holoclar®, approved for use in adult patients with moderate to severe Limbal Stem Cell Deficiency (LSCD) caused by trauma (burns, chemical burns to the eyes). It takes significant effort and time to establish a qualified Holoclar® Treatment Center (HTC). The efficacy of an HTC is assessed based upon the number of treated patients. We evaluated new approaches to improving patient access to the HTC and thus commercial sustainability for Holoclar®.
We mapped new patient pathways and the role of cooperation, networking, coordination among centers and clinical outcomes and focused on the accessibility of Holoclar® across the country. To allow a higher number of patients to be treated, the team successfully implemented a novel approach, patient-doctor tandem travel to utilize an established HTC.
Nine patients were treated with Holoclar®, 3 treated patients came from Brno outpatient clinic, 6 patients had previously been treated in other specialized cornea focus centers and traveled to the HTC in tandem with their attending doctor. After one year of follow-up, there was a remarkable improvement in the monitored parameters in all patients.
The involvement of an external surgery team required careful procedure planning and additional administration. As it is shown in the case of Holoclar® treatment, not only the experts have to be high-skilled and trained, but before the actual treatment can be initiated, the eligible patients have to be identified, evaluated and effectively transferred to the center. Many processes have to be managed and the complexity of this represents a significant barrier to treatment that should not be underestimated. HTC qualification was made possible by the support of the Medasol team facilitating the process and its administration.
Limbal stem cell deficiency; Holoclar®; Qualified treatment center; Personalized medicine; Rare disorders; Tissue engineering; Cell therapy
In this study we concentrated on introducing the complex processing challenges connected to personalized treatment on the example of Holoclar® with the objective of scaling up the access for treatment of a rare disorder [1]. The aim was to identify and test an effective model for the robust use of an advanced therapy. Experience with introducing new personalized medicine, where the manufacturing and treatment process is individual, is limited. Even following an approval of European Medicines Agency (EMA), advanced therapies for rare disorders are not often successfully implemented in standard practice due to three main factors such as eligible patient identification and diagnosis, the complex processing requirements for Cell and Gene Therapies (CGT) and standard coverage of treatment by Health Insurance (HI) [2,3]. The number of small companies entering the field of rare disorders with CGT is increasing significantly, currently 47% of launched products come from small companies [4].
Many of these new small companies are not present in all countries and realize only limited launch activities to introduce new treatments and implement them in standard practice. This tends to inhibit the success of pioneering treatments, which depends heavily on success in establishing new processes under developing regulations, managing administratively demanding activities (logistics, documentation, eligibility, planning of surgery and teams) and building trust among the experts who perform and coordinate the treatment.
In ophthalmology, one such innovative treatment is the cell therapy Holoclar®, approved for use in adult patients with moderate to severe limbal stem cell deficiency caused by trauma, associated with loss of vision in the affected eye in many cases.
Limbal stem cell deficiency is characterized by the loss or deficiency of stem cells in the limbus, which are essential for the renewal of the corneal epithelium and for the barrier function of the limbus. Ocular burns (chemical or physical) may destroy the limbus causing LSCD, a condition that allows bulbar conjunctival cells to invade the corneal surface in an attempt to reform an epithelium. This results in epithelial breakdown and persistent epithelial defects, corneal scarring, neovascularization and chronic inflammation and corneal opacity leading to loss of vision [5-7]. Even though the LSCD is caused by an injury, it is considered a rare disorder. While awareness of this rare disorder is usually high, knowledge of the approved treatment and its accessibility is low.
Holoclar®, registered in the European Union (EU), is a tissue engineering product for advanced therapies consisting of ex vivo reconstructed autologous human corneal epithelium containing stem cells for reconstruction of the corneal surface in patients with LSCD due to ocular burn [8].
The active ingredient of Holoclar® consists of epithelial stem cells which, once rooted, permanently regenerate the corneal epithelium, restoring its full functionality and thus enabling restoration of visual ability.
Current treatment practice
The treatment of LSCD is long-term, very demanding and often yields unsatisfactory results. In the Czech Republic, it consists mainly of supportive treatment to relieve pain and the symptoms of injury (administration of corticosteroids, antibiotics and artificial tears). One procedure that can be performed is allogeneic transplantation of limbal cells, which is perceived to have a relatively low success rate with complications and therefore not performed frequently. Autologous transplantation is performed in a minimum of cases due to its low efficacy and high risks. Other options are repeated corneal transplantations (often with unsatisfactory results), the application of an amniotic membrane or the correction of symblepharon eyelids [9,10].
One recently available option is the ex vivo cultured Limbal Stem Cell (LSC) autologous transplantation treatment Holoclar®, which uses the patient’s own LSCs. The regenerative potential of Holoclar® mainly relies upon the highly proliferative and self-renewing properties of holoclones. Graft transplantation into the injured eye restores the LSC population, allowing a normal transparent corneal surface to be regenerated.
Where treatment with Holoclar® does not result in a satisfactory improvement in visual acuity, further LSCD treatment is available via Penetrating Keratoplasty (PKP). This is undertaken as a second procedure following LSC transplantation in order to replace deep-scarred stroma, with the ultimate aim of restoring sight.
This is a significant development in the field of regenerative medicine; no other stem cell therapy has both met quality standards and demonstrated enough clinical success to achieve authorization status. Holoclar® is currently the only standardized and European Medicines Agency (EMA) approved tissue engineering product [11].
To ensure the best outcome for cell therapy, standardized processes need to be established, including the selection of patients based on complex eligibility criteria, the certification and qualification of the Holoclar® treatment center in line with the Transplantation Centre (TC) and EMA regulations and the standardization of biopsy, manufacturing and implantation processes with follow-up.
Establishing any innovative personalized treatment requires both expertise and interest on the part of medical care providers. The support of hospital management in meeting qualification requirements and maintaining the treatment center is critical.
Geographically, it is important to select a center that can cover a significant part of the population. Since treatment eligible patients tend to be spread in low numbers around specialized centers across the country, it is challenging to set up an effective patient flow. All too often, such specialized centers do not have established processes for patient referrals from other regions and centers.
At present, cornea trauma patient treatment is individual. These patients are treated at regional centers, their traumas and treatment outcomes are specific and their long-term tracking is individual. Additionally, patient groups at cornea centers vary, as different patient groups are treated based on specialists' experience of cornea centers, patients’ trauma and status as well as awareness about Holoclar® innovative treatment. In most cases, patients are referred based on personal requests by attending doctors via an established personal contact with the doctor from the Qualified Treatment Center (QTC).
We evaluated the processes for establishing personalized treatment, the opening and certification of a qualified treatment center and the treatment of 9 patients with LSCD during the period of May 2021 to November 2022.
Selection of the center for Holoclar® certification
The number of eligible patients in cornea centers, expertise and interest, management, validated tissue lab and hospital support were mapped and assessed.
Patient flow management
We mapped the existing practice of referring patients to cornea centers, geographical coverage and consultation set up and proposed solutions to increase patient flow.
Holoclar® treatment
The treatment begins with patient identification via an eligibility evaluation and assessment of comorbidities (Figure 1).
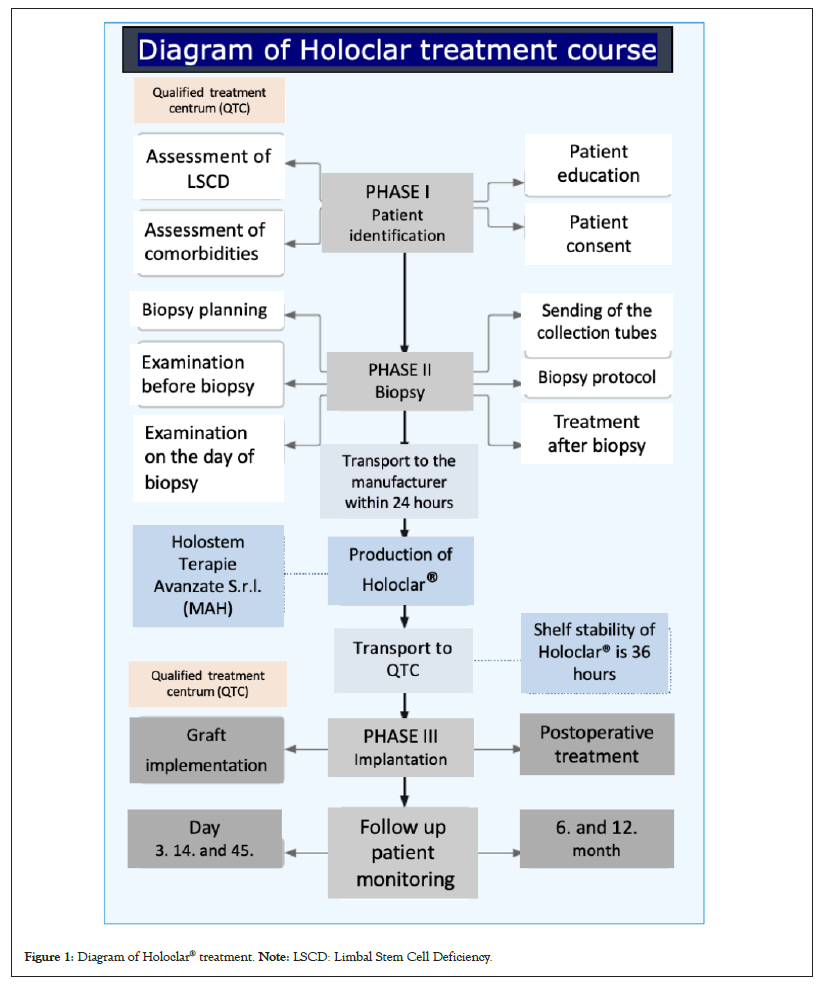
Figure 1: Diagram of Holoclar® treatment. Note: LSCD: Limbal Stem Cell Deficiency.
These include eyelid malpositions, symblephara fornix with fornix shortening, pterygium, severe dry eye syndrome, conjunctival and corneal anesthesia or acute ocular inflammation. These pathologies must be addressed and patients need to meet eligibility criteria prior to biopsy and Holoclar® implantation. Once each patient has been fully informed and given their consent, preparation and planning for biopsy is initiated. Prior to the biopsy, a mandatory initial screening for infectious diseases is conducted. A deep incisional biopsy of 1 mm2-2 mm2 of undamaged limbus (of patient’s healthy part of fellow eye) is taken and transported to the manufacturer Holostem (Market Authorization Holder (MAH)).
Tissue engineering
The manufacturing process is subject to a rigorous production process in line with the Good Manufacturing Practice (GMP) approved by the EMA, with safety controls throughout.
Transplantation surgery starts with very careful, gradual limbal peritomy to minimize bleeding. Then the graft implant is fitted under the undermined conjunctiva. Post-operative treatment consists of an appropriate regimen of topical and systemic anti-inflammatory, prophylactic antibiotic treatment and monitoring schedule.
Detailed information regarding the treatment is provided as a standard part of training support for every qualified center and Holoclar® qualified team. These materials are available on request.
Patient group
In this study we report on 9 patients who met eligibility criteria consisting of clinical status evaluation and standardized screening; (age 28 yrs-63 yrs), 8 men and 1 woman, with 7 cases of alkali burn and 1 acid burn case and 1 case of physical burns trauma that led to LSCD as shown in Table 1. All 9 patients underwent a comprehensive preoperative examination with assessment of the degree of LSCD and assessment of concomitant diseases contraindicated for Holoclar® implantation.
| Patient number | Age | Type of injury | Time since the injury/months | Cornea center vascularization 6 mm | Cornea center transparency 6 mm | Quadrants of vascularization | Previous operations | Visual acuity |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | Alkali burn | 84 | Yes | No | 4 | ALSCT | 0.03 |
| 2 | 31 | Alkali burn | 180 | Yes | No | 4 | Artephakia, express implantant 2x, DMEK, DSEK, PKP 2x | HM |
| 3 | 28 | Physical burn | 36 | Yes | No | 4 | Amniotic membrane, ALSCT | 0.06 |
| 4 | 46 | Acid burn | 85 | Yes | No | 4 | Amniotic membrane 4x | LP, HM |
| 5 | 63 | Alkali burn | 95 | Yes | No | 4 | Amniotic membrane 3x, ALSCT | 0.1 |
| 6 | 39 | Alkali burn | 37 | Yes | No | 4 | No | LP, HM |
| 7 | 55 | Alkali burn | 192 | Yes | No | 4 | No | HM |
| 8 | 51 | Alkali burn | 176 | Yes | No | 3 | Amniotic membrane 6x, PKP 2x | HM |
| 9 | 35 | Alkali burn | 121 | Yes | No | 4 | Amniotic membrane | 0.1 |
Note: LP: Light Perception; HM: Hand Movement; ALSCT: Autologous Limbal Stem Cell Transplantation; PKP: Penetrating Keratoplasty; DMEK: Descemet Membrane Endothelial Keratoplasty; DSEK: Descemet Striping automated Endothelial Keratoplasty.
Table 1: Patient status profile before Holoclar® treatment.
The patients had suffered LSCD for 36-192 months prior to the commencement of Holoclar® treatment. Five out of 9 patients reported visual acuity only of Hand Movement (HM) and 4 reported visual acuities between 0.03-0.1. All patients had no cornea transparency and most had 4-quadrant vascularization, including in the central area of the cornea. Seven out of 9 patients had previously undergone several (over 27 in total) corrective surgeries. 4 patients had undergone more than 4 corrective surgeries each (Table 1).
Evaluation criteria of clinical outcome
Each patient’s status profile was evaluated before and immediately after the graft implant and one year after the implant as shown in Table 2. The evaluated criteria were type of injury, time since injury, visual acuity, cornea center transparency, vascularization, previous operations, perioperative complications, planned and performed further surgeries and treatment evaluation. The efficacy of treatment was assessed based on the criteria such as improvement of vascularization and of cornea center transparency, visual acuity and occurrence of complications.
| Patient number | Cornea center vascularization 6 mm | Cornea center transparency 6 mm | Quadrants of vascularization | Performed surgeries | Planned further surgeries | Visual acuity |
|---|---|---|---|---|---|---|
| 1 | No | Partial | 3 | No | No | 0.1 |
| 2 | No | Partial | 3 | No | PKP | 0.02 |
| 3 | No | Partial | 3 | No | No | 0.1 |
| 4 | No | Partial | 3 | No | No | 0.05 |
| 5 | No | Yes/after PKP | 2 | PKP | - | 0.15/after PKP |
| 6 | No | No | 3 | No | PKP | 0.05 |
| 7 | No | Partial | 3 | No | PKP | 0.1 |
| 8 | No | No | 1 | No | No | HM |
| 9 | No | Yes | 0 | No | No | 0.4 |
Note: HM: Hand Movement; PKP: Penetrating Keratoplasty.
Table 2: Clinical outcomes of patients 1 year after Holoclar® treatment.
Evaluation of central cornea (6 mm in diameter) vascularization and transparency status was conducted according to the Holoclar® protocol. Corneal vascularization was assessed by 2 doctors and where the assessment outcome was not consistent, a 3rd opinion was requested and the average of the outcome reported.
Eligibility for reimbursement
The reimbursement of the Holoclar® treatment was approved, but with indication criteria that were narrower than in registration. An individual request was submitted to HI for review and reimbursement approval.
The team wanted both to assess the ability to implement and incorporate personalized medicine within the framework of existing processes and to evaluate healthcare providers’ need, willingness and ability to introduce new and effective approaches allowing a higher number of patients to be treated with high clinical efficacy and sustainability.
The clinical outcomes with Holoclar® are only partially shared, since the excellent results exceed the scope of this publication and will be published as a standalone contribution.
Holoclar® treatment center selection and qualification
Introduction of Holoclar® treatment was planned in the Czech Republic, but certification of the pre-selected center had not yet been completed in 2020. Holostem, the Market Authorization Holder (MAH) for Holoclar®, assigned Bluedil, which in turn appointed Medasol (a partner with knowledge of regulations, launch and commercialization expertise for rare disorders including central European market) to make the treatment accessible to patients in the Czech Republic. Medasol assessed the situation and focused on selecting the optimal treatment center, then followed with qualification procedures.
The Czech Republic has 9 cornea centers at 12 university hospitals and several at private clinics, but it was considered reasonable to open only 1-2 Holoclar® treatment centers in the country due to the low number of patients and because it is a highly demanding process to qualify and maintain the treatment center.
From 9 centers Medasol team narrowed down the selection to 3 centers such as Charles University and General University Hospital in Prague (Prague VFN), Charles University and University Hospital Kralovske Vinohrady (Prague FN KV) and University Hospital Brno (FN Brno) (Figure 2).
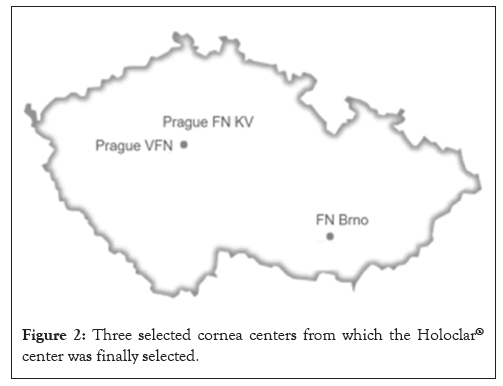
Figure 2: Three selected cornea centers from which the Holoclar® center was finally selected.
The geographical coverage and bigger population counted in favor of selecting a center in Prague, but in Brno the benefits of supportive hospital management and other aspects outweighed its less advantageous geographical location.
Despite the smaller number of patients connected with FN Brno (in comparison to the Prague centers), FN Brno was finally selected for completion of the qualification, based on the fact that it was the furthest in the qualification assessment process and for its readiness to complete agreements such as those required for legal and financial compliance. In favor of FN Brno was also the availability of the certified tissue lab. In FN Brno, the hospital management was the most supportive, showing interest and matrix support that matched the interest of their experts, which was important both for the opening and the long-term maintenance of the treatment center.
When all agreements were signed, the qualified Holoclar® treatment center was successfully opened.
Patient flow
Then the team focused on the accessibility of Holoclar® across the regions and country, increasing the patient flow. The majority of patients were diagnosed in local ophthalmology clinics and assessed for eligibility at regional cornea centers with larger catchment areas and appropriate expertise. The qualified center in Brno identified patients arriving from close surroundings, but the number was low and could be only increased by networking with other centers. The new patient pathway, the importance and role of cooperation, network and coordination among centers were mapped. All centers could send patients to the Holoclar® center directly and without delays or constraints.
Since most patients were located and had initially been treated in Prague, a novel approach was proposed and successfully arranged, a patient-doctor tandem travel between the Prague centers and Brno QTC.
Overall, both doctors and patients demonstrated a willingness to commence treatment, even amid the heightened restrictions imposed by Coronavirus Disease (COVID). In a majority of instances, individuals were prepared to travel over 250 kilometers each way to and from the center multiple times over the six-month period. The result of the study, mainly the number of the treated patients, was also influenced by COVID related regulations.
We evaluated the accessibility of Holoclar® to patients with LSCD. Out of 91 patients initially diagnosed and identified, 9 were identified as eligible and were treated with Holoclar®. Surprisingly, it was found that despite being listed in outpatient clinic files, 50 patients could not be re-contacted. Three out of 9 treated patients came from Brno outpatient clinic and 6 patients had previously been treated in Prague specialized cornea focus centers. Six out of 9 treated patients traveled to the qualified center in tandem with their attending doctor or surgeon. Thanks to the new tandem model, the number of patients accessing treatment increased from 3 to 9 patients.
Biopsy outcome, tissue engineering outcome
For the treatment a deep biopsy of 1 mm²-2 mm² of undamaged limbus is required. A total of 10 biopsies were withdrawn for tissue engineering, since in one patient the biopsy needed to be repeated. The epithelial cells were identified. The percentage of cells endowed with regenerative capacity (showing viability and active functions for tissue regeneration) was 20.9% (mean).
The number of regenerative cells doubled after culture, enabling whole-tissue reconstruction. The number of stem cells obtained exceeded the specified minimum required for optimal biopsy withdrawal, correct patient selection and corneal maintenance in the long-term [12,13].
Two of the batches were below 2% of stem cells, but one of them was transplanted, as the tissue had appropriate quality criteria for the specific patient condition. The regenerative capacity of each biopsy, after extraction, ranged from 9% to 42% of the total cells (20.9% mean). The regenerative capacity rose during the culture process, with 43.6% (range 14%-78%) of cells ultimately exhibiting the ability to regenerate a part of the tissue over time. The withdrawal of biopsy and the culture allowed a retrieval of a number of stem cells sufficient for the long-term maintenance of the released tissues, with a mean value of 2.9% (range 2.5%- 5.6%).
Clinical outcome
Implant of each graft was organized with an expert surgery team once the batch was released and batch delivery specified. Limbal peritomy and implantation was performed without complication.
Patients were monitored to detect potential infection, secondary glaucoma and dry eye. After graft implantation, 1 patient had transient dysfunction of the express implants. No other short or long-term complications were reported. Postoperative care and follow up was scheduled according to protocol.
Clinical outcome after 1 year
After one year of follow-up, there was a significant improvement in the monitored parameters in all patients as shown in Table 2. The central 6 mm optic zone is avascular in the entire set. Quarters of perilimbal vascularization decreased in all patients. The transparency of the center of the cornea improved in 7 patients and visual acuity in 8 patients. One patient has already undergone PKP and 3 patients are scheduled for PKP. Other patients have declared themselves to be satisfied with their current status so far and their need for PKP will be reevaluated in the future (Figure 3 and Table 2) [14-17].
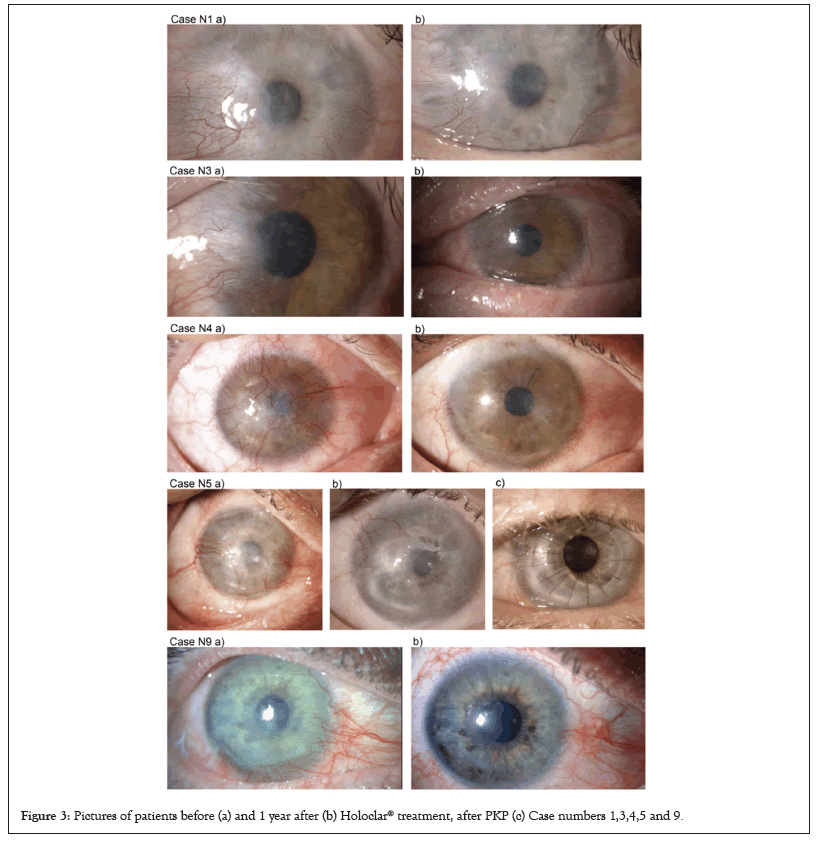
Figure 3: Pictures of patients before (a) and 1 year after (b) Holoclar® treatment, after PKP (c) Case numbers 1,3,4,5 and 9.
Experts observed improvements in the critical area of the central cornea, measured by transparency and vascularization. Nine out of 9 patients had no sign of central cornea area vascularization. Seven out of 9 had improved transparency of the central area of the cornea. Quadrants of vascularization decreased in all patients and vascularization was more located at the limbus. In all patients, thanks to improvements at the cornea center, visual acuity was found to have improved. All patients reported that they had no discomfort (pain) and that irritation had subsided. Their condition is still improving with time.
The main focus of this study was to identify pathways and stumbling blocks to the implementation of personalized medicine into standard practice. Practical experience proved necessary to explore novel ways to introduce and sustainably maintain advanced treatment availability. The individual, case-by-case profile of innovative treatments is lengthy and usually takes more than 12 months of treatment with several visits. This investment must be measured against the high value of the treatment, which in this case meant restoring sight.
Damage to the corneal limbal cells by severe chemical and physical injury, inflammation and congenital diseases, leads to LSCD. This causes loss of corneal regeneration with persistent epithelial defects, vascularization, scarring and loss of corneal transparency [18-20]. Perforating keratoplasty is contraindicated in these cases. Until now, treatment consisted of the application of amniotic membrane (in the early stages), autologous limbal cell transplantation in cases of unilateral involvement or allogeneic transplantation in cases of bilateral involvement, but this required immunosuppressive treatment [21,22]. Ex vivo culture of autologous limbal stem cells as Cultivated Limbal Epithelial Transplantation (CLET) for treatment of ocular surface was introduced in 1997 by Pellegrini, et al., [12]. The technique can be used with great success in both unilateral and partial bilateral LSCD. It can be limited by the price and above all by the technical readiness and experience of the technical engineering. Holoclar® implantation leads to restoration and stabilization of the corneal surface. It improves corneal transparency, visual acuity and optimizes corneal preparation for possible perforating keratoplasty.
No complications (infection, secondary glaucoma, dry eye syndrome) were observed and patients reported improvement and pain/discomfort relief. The center of the cornea was without vascularization, mostly transparent. Eight out of 9 patients reported restoring vision, with further improvement with PKP in one patient (to date). Surgeons reported further improvement over time, with gradual and ongoing smoothing of the surface of the treated eye. Holoclar® can further be considered as a suitable preparation prior to performing PKP. The clinical results of Holoclar® treatment are therefore very encouraging, demonstrating an important progress in a challenging treatment of LSCD.
Prior to the onset of treatment with Holoclar®, all 9 patients in this study had reported ongoing discomfort and pain and a continued need for advanced treatment despite 37-192 months of intense treatment and 27 interventions in total. The good outcomes for Holoclar® indicate that this remains a viable therapy even after such invasive surgery as failed Conjunctival Limbal Autograft (CLAU) and it may provide a treatment pathway for patients not included in Simple Limbal Epithelial Transplantation (SLET) selection due to total LSCD and deep stromal damage [23- 27]. Those patients can recover visual acuity after PKP without compromising the previous stem cell transplant.
The treatment of LSCD is very complex. Initial results show good outcomes for treatment with Holoclar®, but treatment remains a medically, legislatively and financially demanding process necessitating interdisciplinary cooperation and support.
Clinical expertise is crucial, but nonetheless still only a part of a bigger picture of the treatment process. For each individual patient over 50 qualified personnel, all of whom underwent specific training, contributed in a coordinated process to successful treatment. Each team member had a specific role and responsibility at a given time, guaranteeing the outcome by their signature.
Certification of a qualified treatment center demands investments in time and dedication, training and equipment and the accompanying documentation is demanding. The regulatory obligations (for CGT especially) are gradually increasing, pushing all parties to incorporate higher standards and qualifications to achieve better treatment outcomes. When looking for efficiencies in establishing and supporting a QTC, it is useful to appoint an experienced partner. In this case, partnership with Medasol helped reduce the time to qualification from years to months.
When selecting a QTC, it is critical to evaluate the current treatment skills of the center as well as patient pathways and accessibility. The effective management of the center and its readiness to proactively address legal, financial and qualitative requirements are critical. To allow a higher number of patients to be treated, consider new cooperative frameworks and contracting additional remote experts.
Medasol team proposed and successfully implemented a novel approach; the allowance of patient-doctor tandem, which represented the biggest group of patients. The trained specialists and surgeons, who traveled together with their patients, could utilize the certified facility and share experience with Brno QTC experts, creating a hub of excellence that consisted of established processes and trained, qualified team. It enlarged the qualified and trained Holoclar® team.
The number and interchangeability of trained experts plays an important role in planning treatment and in addressing short-term team changes (sickness, urgent circumstances) and long-term changes (maternity leave, relocation). It applies for all process-specified team members (surgeons, nurses, tissue lab team, pharmacy and qualified person, etc.,) as well. The flexibility to find solutions during challenging times or team changes is required to maintain the accessibility to treatment. The investment involved in qualifying as a specialized treatment center should be balanced by treating as high a number of patients as possible, so securing a stable patient flow to the treatment facility is a vital part of the long-term success of high-value personalized treatment. When looking for efficiency, an experienced partner such as Medasol to certify and maintain the HTC may prove invaluable.
Eligibility for reimbursement and the market access issue
It must be noted that personalized medicine is expensive. In general, market access for CGT is challenging, but not prohibitive, as evidenced by the fact that at least 3 successful CGTs are used in practice globally [28]. In the case of Holoclar®, its high value is undisputed, since it permits patients to recover lost sight.
In the market context of this study, reimbursement for the Holoclar® treatment was approved by the health insurance provider after lengthy discussions, but with indication criteria that were narrower than those clinically required for registration. However, due to the nature of biopsy, the quality of entry biopsy materials varies. The tissue engineering manufacture process is designed to obtain material within the quality range defined for batch release. As a result of the Health Insurance (HI) provider setting even narrower requirements in the reimbursement criteria, some batches could not be used while being covered by reimbursement [29,30]. This could raise ethical issues which are novel and connected solely to pioneering treatments. Hitherto, reimbursement frameworks have been based on the most common treatment models (especially drugs), but authorities are working on updating regulations to accommodate new treatment advances such as CGTs. With an increasing need for more effective treatment solutions, new individual and systematic approaches for CGT reimbursement are being implemented. Medasol successfully reached agreements with HIs and hospitals that allowed access to reflect the variability of biopsy and tissue engineering and in line with registration. Treatments were fully reimbursed for all patients.
With the increasing desire for more effective treatments and an increased number of approved CGTs, we can anticipate clearer entry criteria and faster acceptance of CGT into practice. Today 3,951 therapies are in development from the preclinical to pre-registration stage, of which 53% are CGT. Holoclar® becoming the first cell therapy approved for LSCD in 2014. We have had the opportunity to observe the implementation of personalized CGT medicine in practice. Holoclar® outcomes met expectations of doctors and patients. These results are encouraging when implementing personalized cell treatment in practice.
We wish to extend our gratitude to our colleagues from the tissue lab, RNDr. Rita Pacasova and Mgr. Simona Michlickova, for their valuable cooperation in establishing and maintaining needed processes. We also thank PharmDr. Hana Adlerova and her team, Mgr. Lenka Rosenbaumova from the legal department, MUDr. Eva Tesarova and Mgr. Vaclav Zivec from the financial department at FN Brno for establishing new approaches, contracts and innovative solutions to allow the use of Holoclar® in practice and Denisa Janova for initiating the project.
Holostem was partnered with Bluedil in Europe and Medasol s.r.o. as a partner of Bluedil to represent Holostem in the Czech Republic. Medasol has experience in the commercialization of orphan drugs in central Europe and its activities primarily help to make treatment accessible to patients with serious and rare diseases.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Marinova C, Vlkova E, Horackova M, Netukova M, Skalicka P, Pellegrini G, et al (2024) Optimizing Vision Restoration: Experience with Holoclar Treatment, the Selection of a Qualified Treatment Center, Accessibility to Patients and Outcomes. J Clin Exp Ophthalmol. 15:971.
Received: 20-Feb-2024, Manuscript No. JCEO-24-29695; Editor assigned: 22-Feb-2024, Pre QC No. JCEO-24-29695 (PQ); Reviewed: 07-Mar-2024, QC No. JCEO-24-29695; Revised: 14-Mar-2024, Manuscript No. JCEO-24-29695 (R); Published: 21-Mar-2024 , DOI: 10.35248/2155-9570.24.15.971
Copyright: © 2024 Marinova C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.