Angiology: Open Access
Open Access
ISSN: 2329-9495
+44 1478 350008
ISSN: 2329-9495
+44 1478 350008
Research Article - (2024)Volume 12, Issue 12
Background: The impact of Guanylate Cyclase (sGC) stimulators on clinical outcomes of HF patients were still controversial.
Objectives: To investigate the efficacy and safety of oral soluble sGC stimulators in Heart Failure (HF).
Methods: PubMed, Web of Science, and Cochrane Library were searched to identify Randomized Controlled Trials (RCTs) comparing sGC stimulators vs placebo in HF patients. A total of 6 RCTs with 7382 HF patients were included.
Results: Compared with placebo, oral sGC stimulators reduced the risk of a composite of Cardiovascular (CV) death or Hospitalization for HF (HHF), Odd’s Ratio (OR) 0.87, 95% Confidence Interval (CI) 0.78-0.98, p=0.02), and CV death [OR 0.45, 95% CI 0.38-0.53, p<0.001]. Significant reduction in the CV death [OR 0.42, 95% CI 0.35-0.50, p<0.001] of Heart Failure with Reduced Ejection Fraction (HFrEF) was also found in sGC stimulator group. However, certainty of evidence evaluated by Grading of Recommendations Assessment, Development, and Evaluation pro Guideline Development Tool (GRADEproGDT) was moderate or low for the main efficacy outcomes. There was no significant difference between sGC stimulators and placebo in adverse events [OR 0.97, 95% CI 0.86-1.09, p=0.64] or serious adverse events [OR 0.92, 95% CI 0.83-1.03, p=0.14].
Conclusions: Oral sGC stimulators may be associated with a lower risk of CV death or HHF, but the certainty of evidence in this meta-analysis was low. Large-scale, multicenter RCTs with enough statistical power for “hard” efficacy outcomes are urgently needed to determine the impact of sGC stimulators on prognosis of HF.
Oral sGC stimulators; Heart failure; Meta-analysis
6MWD: 6-Minute Walk Distance; AE: Adverse Event; cGMP: cyclic Guanosine Monophosphate; CSS: Clinical Summary Score; CV: Cardiovascular; HF: Heart Failure; HHF: Hospitalization for Heart Failure; HFpEF: Heart Failure with Preserved Ejection Fraction; HFrEF: Heart Failure with Reduced Ejection Fraction; KCCQ: Kansas City Cardiomyopathy Questionnaire; MRA: Mineralocorticoid Receptor Antagonist; NO: Nitric Oxide; OSS: Overall Summary Score; RAAS: Renin–Angiotensin–Aldosterone System; PAH: Pulmonary Arterial Hypertension; RCT: Randomized Controlled Trial; PLS: Physical Limitation Score; SAE: Serious Adverse Event; sGC: soluble Guanylate Cyclase; SGLT-2: Sodium‐Glucose cotransporter‐2; TSS: Total Symptom Score
With the advance of medical therapy, the prognosis of HF has been improved. Currently guidelines recommend a new quadruple therapy, including Renin–Angiotensin–Aldosterone System (RAAS) inhibitor, β receptor blocker, Mineralocorticoid Receptor Antagonist (MRA), Sodium‐Glucose cotransporter‐2 (SGLT-2) inhibitor as first-line treatment for HF [1-3]. Despite such great progress, 5-10% of HF patients still devastate to end-stage heart failure in each year, with a mortality of 50% [4]. Therefore, the continuous exploration of novel treatments for HF should not stop.
sGC stimulators is an enzyme in the Nitric Oxide (NO)-sGC-cyclic Guanosine Monophosphate (cGMP) pathway [5,6]. Due to its pharmacological effects of promoting vasodilation, inhibiting vascular remodeling, and reducing inflammatory responses, sGC stimulators are widely used in the treatment of Pulmonary Arterial Hypertension (PAH) [7,8]. In recent years, several RCTs have been conducted to explore the effect of sGC stimulators in HF patients [9-14]. However, due to the between-study population heterogeneity, limited sample size and distinct results, the impact of sGC stimulators on clinical outcomes of HF patients was still controversial. We therefore conducted a meta-analysis of RCTs exploring the efficacy and safety of sGC stimulators in HF, in order to determine the role of sGC stimulators on the prognosis of patients with HF.
Data sources and search strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15,16] The GRADE approach were used to evaluate the certainty of evidence [17]. A systematic literature search was performed on June 10, 2023, using PubMed, Embase, and Web of Science databases. The search strategy included the following keywords: soluble guanylate cyclase stimulator, sGC stimulator, vericiguat, riociguat, praliciguat and heart failure. Only English literature and full manuscripts were included, and abstracts were excluded.
Inclusion criteria: (i) RCTs recruiting patients diagnosed with HF; (ii) RCTs comparing clinical outcomes between sGC stimulators and placebo or other medications.
Exclusion criteria: (i) Studies recruiting patients with PAH; (ii) Observational or non-randomized studies; (iii) Studies that did not report clinical outcomes.
Study selection and eligibility criteria
Data extraction and outcomes: Data extraction was performed by independently two authors Qiang Ren and Sicong Ma. Extracted information includes authorship, country, study design, number of patients, age, sex, information regarding patient diagnoses, clinical information and medications, classification of heart failure, primary study endpoints and other endpoints. After screening titles and abstracts to remove irrelevant articles, we reviewed full texts of the remaining potential articles. Risk of bias assessment was performed according to the Cochrane Risk of Bias assessment tool (RoB 2.0) [18]. Any discrepancies in data extraction or quality assessment were resolved by a third reviewer Quanyu Zhang. In this study, we also applied the GRADE method in order to assess the quality of the evidence for each outcome, GRADE method can be found and accessed in GRADEpro GDT software. The primary outcome was a composite of CV death or HHF. Secondary efficacy outcomes are CV death, HHF, all-cause death, 6-Minute Walk Distance (6-MWD), Kansas City Cardiomyopathy Questionnaire (KCCQ, range 0-100, higher values indicate better functioning, including Total Symptom Score (TSS), Clinical Summary Score (CSS), Overall Summary Score (OSS) and Physical Limitation Score (PLS). The safety outcomes include any Adverse Event (AE), such as any study drug-related AE and AE with the outcome of death; any Serious Adverse Event (SAE), including any study drug-related SAE.
Statistical analysis
Mean and standard deviation were calculated for estimates of continuous variables. OR with 95% CI were calculated to estimate efficacy and safety outcomes. For trials involving multiple intervention groups, a formula was used to amalgamate the mean and standard deviation of each group into a single entity. To evaluate between-study heterogeneity in the meta-analysis, the heterogeneity statistic (I²) was used. A random-effects Mantel-Haenszel (M-H) model was employed. Publication bias was assessed using funnel plots and asymmetry test with a p<0.1 indicating publication bias existed. A two-tailed p-value<0.05 was considered statistically significant for outcome analyses. Subgroup analysis was conducted based on the classification of HFpEF and HFrEF. Statistical analyses were conducted using Review Manager (RevMan) 5.3 and R (programming language) 4.3.1.
Study characteristics
361 records were initially searched. After removal of irrelevant and duplicate items, 42 studies had remained for full-text and reference review. Of these, 36 articles were excluded, and 6 articles were finally included for quantitative analysis [9-14]. The search and study selection process of this meta-analyses was summarized in the flowchart Figure 1. A total of 7382 HF patients were included in our meta-analysis. Table 1, showed the baseline characteristics of the included trials. 2 studies [11,13] recruited with HFrEF, and the other 4 studies enrolled HFpEF [9,10,12,14]. The study drug of most trials was vericiguat, except for one study, of which praliciguat was the study drug.
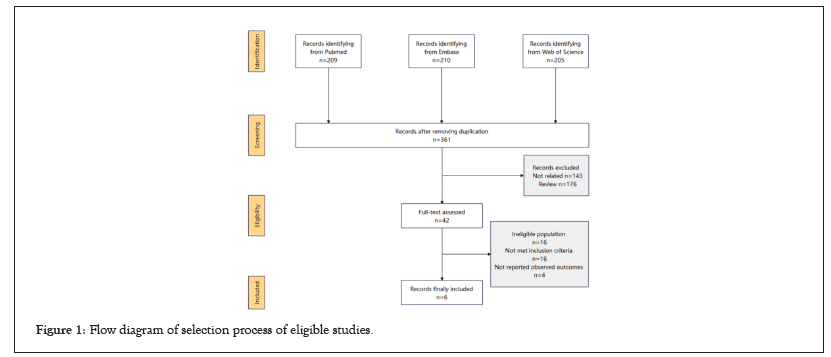
Figure 1: Flow diagram of selection process of eligible studies.
| Study ID | Udelson et al. [8] | Pieske et al. [9] | Gheorghiade et al. [10] | Filippatos et al. [11] | Armstrong et al. [12] | Armstrong et al. [13] | ||||||
| Population characteristics | HFpEF ≥ 40 (181) | HFpEF ≥ 45 (477) | HFrEF ≤ 45 (456) | HFpEF ≥ 45 (429) | HFrEF ≤ 40 (5,050) | HFpEF ≥ 45 (789) | ||||||
| Follow-up duration | 12 Weeks | 12 Weeks | 16 Weeks | 12 Weeks | 12 Months | 24 Weeks | ||||||
| Drug | Praliciguat (40 mg) |
Placebo | Vericiguat (1.25 mg/2.5 mg/2.5-5 mg/2.5-10 mg) |
Placebo | Vericiguat (1.25 mg/2.5 mg/2.5-5 mg/2.5-10 mg) |
Placebo | Vericiguat (1.25 mg/2.5 mg/2.5-5 mg/2.5-10 mg) |
Placebo | Vericiguat (10 mg) |
Placebo | Vericiguat (15 mg/10 mg) |
Placebo |
| Age, years | 70.7 (9.2) | 70.1 (9.0) | 73 (10) | 74 (9) | 68 (12.25) | 67 (13) | 73.02 (9.85) | 74 (9.1) | 67.5 (12.2) | 67.2 (12.2) | 72.65 (9.4) | 72.8 (9.4) |
| Diabetes, N (%) | 46 (50.5) | 50 (55.6) | 185 (48) | 47 (50.5) | 178 (49) | 41 (44.6) | 165 (49) | 47 (50.5) | 1226 (48.6) | 1143 (45.3) | 117.5(44.61) | 123 (46.9) |
| Coronary artery disease,N (%) | 36 (39.6) | 35 (38.9) | NA | NA | NA | NA | 136 (40.48) | 43 (46.2) | 1511 (59.8) | 1433 (56.8) | 117.5 (44.61) | 127 (48.5) |
| Hypertension, N (%) | 90 (98.9) | 87 (96.7) | 347 (90) | 85 (91.4) | 287 (79) | 70 (76.1) | 245 (72.92) | 72 (77.4) | 2002 (79.3) | 1993 (79.0) | 243 (92.2) | 243 (92.7) |
| NT-proBNP, pg/ml | 232 | 215.5 | 1,157 | 2,254 | 2,763 | 4043 | NA | NA | 2803.5 | 2821 | 1351.8 | 1644.2 |
| (133-490) | (93-501) | (397-2,611) | (932-3,644) | (1,491.5-7,318.5) | (1,962-6,712) | (1,572-2,821) | (1,548-5,206) | (636.9-2,934.5) | (795.8-3,161.2) | |||
| NYHA III/IV, N (%) | 28 (30.8) | 24 (26.7) | NA | 39 (41.9) | 178 (49) | 38 (41.3) | NA | NA | 1045 (41.4) | 1024 (40.6) | 110.5 (41.9) | 106 (40.5) |
| ACEI or ARB or ARNI,N (%) | 27 (29.7) | 36 (40.0) | 281 (69) | 40 (43.0) | 311 (85) | 73 (79) | NA | NA | 1847 (73.3) | 1853 (73.6) | NA | NA |
| Beta blocker, N (%) | 39 (42.9) | 24 (26.7) | 304 (79) | 76 (81.7) | 328 (90) | 83 (90.2) | NA | NA | 2349 (93.2) | 2342 (93.0) | NA | NA |
| MRA, N (%) | NA | NA | 139 (36) | 39 (41.9) | 234 (64) | 50 (54.3) | NA | NA | 1747 (69.3) | 1798 (71.4) | NA | NA |
| SGLT-2 inhibitor, N (%) | 2 (2.2) | 1 (1.1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Note: HFrEF: Heart Failure with Preserved Ejection Fraction; HFpEF: Heart Failure with Preserved Ejection Fraction; NT-proBNP: N-Terminal pro-Brain Natriuretic Peptide; ACEI: Angiotensin Converting Enzyme Inhibitor; ARB: Angiotensin Receptor Blocker; ARNI: Angiotensin Receptor-Neprilysin Inhibitor; MRA: Mineralocorticoid Receptor Antagonist; NA: Not Applicable.
Table 1: Characteristics of the clinical trials and patients included in the meta-analysis.
One trial did not explicitly elucidate the blinding of outcomes assessment [14]. The outcomes of this study were not analyzed in accordance with a pre-defined analysis. Therefore, this study was regarded as “high” of ROB, and the other five trials as “low” Supplementary Figure 1. Funnel plots did not show any publication bias Supplementary Figures 2-5.
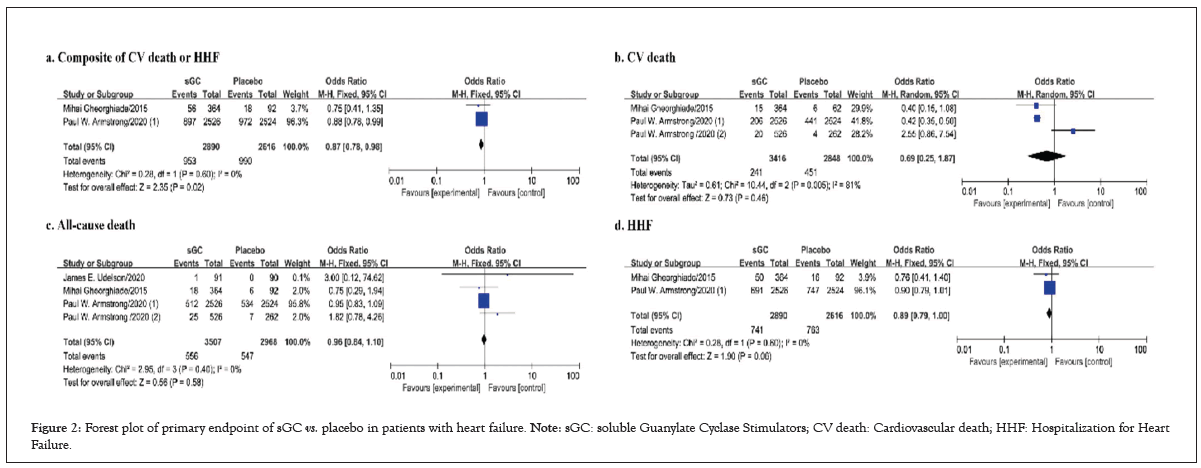
Figure 2: Forest plot of primary endpoint of sGC vs. placebo in patients with heart failure. Note: sGC: soluble Guanylate Cyclase Stimulators; CV death: Cardiovascular death; HHF: Hospitalization for Heart Failure.
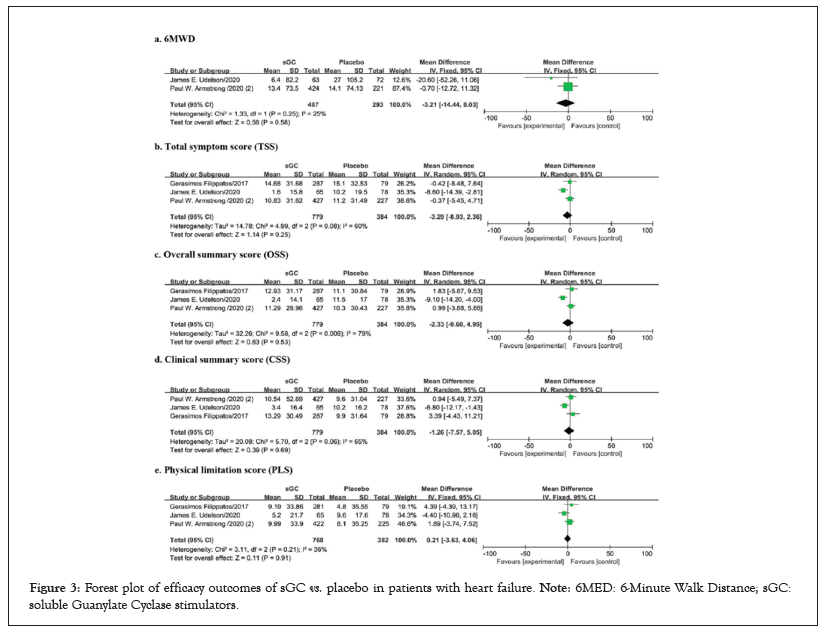
Figure 3: Forest plot of efficacy outcomes of sGC vs. placebo in patients with heart failure. Note: 6MED: 6-Minute Walk Distance; sGC: soluble Guanylate Cyclase stimulators.
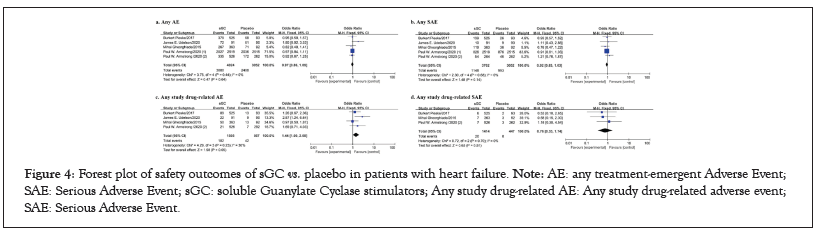
Figure 4: Forest plot of safety outcomes of sGC vs. placebo in patients with heart failure. Note: AE: any treatment-emergent Adverse Event; SAE: Serious Adverse Event; sGC: soluble Guanylate Cyclase stimulators; Any study drug-related AE: Any study drug-related adverse event; SAE: Serious Adverse Event.
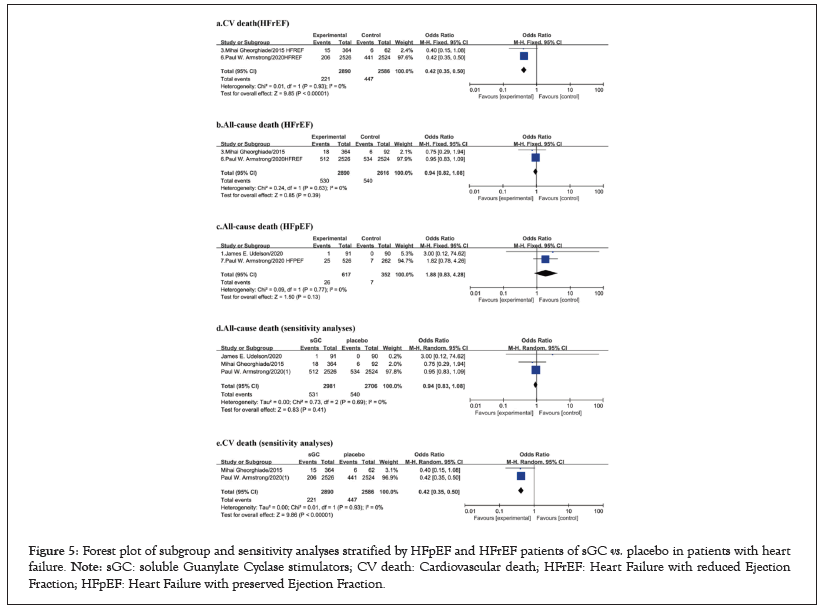
Figure 5: Forest plot of subgroup and sensitivity analyses stratified by HFpEF and HFrEF patients of sGC vs. placebo in patients with heart failure. Note: sGC: soluble Guanylate Cyclase stimulators; CV death: Cardiovascular death; HFrEF: Heart Failure with reduced Ejection Fraction; HFpEF: Heart Failure with preserved Ejection Fraction.
Outcomes for the overall HF patients
Only two studies reported the primary outcome, a composite of CV death or HHF. Compared with placebo, oral sGC stimulators significantly reduced the risk of composite of CV death or HHF (I²=0%, OR 0.87, 95%CI 0.78-0.98, p=0.02 (Figure 2a). For CV death from 3 studies, oral sGC stimulators significantly reduced the rate of CV death in comparison with placebo (I²=81%, OR 0.45, 95%CI 0.38-0.53, p<0.001; Figure 2b). However, no significant difference in all-cause death (I²=0%, OR 0.96, 95%CI 0.84-1.10, p=0.58; Figure 2c) or HHF (I²=0%, OR 0.89, 95% CI 0.0.79-1.00, p=0.06; Figure 2d) was observed between placebo and sGC (Figure 2).
For the analysis of quality of life, articles involving a total of 1163 patients were included in the quantitative analysis. No differences were found in 6MWD, KCCQ TSS, KCCQ OSS, KCCQ CSS, and KCCQ PLS (p>0.05 for all, between oral sGC stimulators and placebo (Figure 3). These results may be compromised due to the limited number of included studies and potential publication biases in outcomes of quality of life. 5 studies, with a total of 7076 patients were included for the quantitative analysis of safety outcomes. No significant differences between SGC stimulators and placebo were found in safety outcomes, including any AE, any SAE, any study drug-related AE, any study drug-related SAE (p>0.05 for all) (Figure 4).
Subgroup and sensitivity analyses stratified by HFpEF and HFrEF patients
We conducted subgroup analyses stratified by HFrEF and HFpEF patients. The HFrEF subgroup included 2 studies with a total of 5506 patients, and HFpEF subgroup included 4 articles with 1876 patients (Figure 5). The results showed that oral sGC stimulators significantly reduced the rate of CV death (I²=0%, OR 0.42, 95%CI 0.35-0.50, p<0.001; Figure 5a) compared to placebo in HFrEF patients. However, there was no difference in all-cause death between oral sGC stimulators and placebo in either HFrEF patients (I²=0%, OR 0.94, 95%CI 0.82-1.08, p=0.39; Figure 5b) or HFpEF patients (I²=0%, OR 1.88, 95%CI 0.83-4.28, p=0.13; Figure 5c).
Armstrong et al., was regarded as “high” of ROB [13]. Therefore, we conducted the sensitivity analyses after excluding this study. Sensitivity analysis found that oral sGC stimulators reduced the risk of composite of CV death (I²=0%, OR 0.42, 95%CI 0.35-0.50, p<0.01; Figure 5b), which was consistent with the main outcome. No differences were found in other efficacy or safety outcomes between two groups (Figure 5d and 5e).
Certainty of evidence
The certainty of evidence evaluated by the GRADEproGDT was shown in Table 2. The GRADE evidence for composite of CV death or HF hospitalization, all-cause death, HF hospitalization, CV death of HFrEF patients, all-cause death of HFrEF patients, any adverse event, any serious adverse event, any serious adverse event and any serious adverse event delivery outcomes is of moderate quality (Figure 6).
| Quality assessment | No of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | sGC | placebo | Relative | Absolute | ||
| (Treatment) | (control) | (95% CI) | (95% CI) | |||||||||
| Composite of CV death or HF hospitalization | ||||||||||||
| 2 | randomized trials | not seriousa | seriousb | not serious | not serious | none | 953/2890 | 990/2616 | OR 0.87 | 32 fewer per 1000 | ⊕⊕⊕ moderate |
critical |
| -33% | -37.80% | (0.78 to 0.98) | (from 5 fewer to 56 fewer) | |||||||||
| CV death | ||||||||||||
| 3 | randomized trials | not serious | very serious | not serious | seriousc | none | 241/3416 | 451/2848 | OR 0.45 | 80 fewer per 1000 | ⊕ very low |
critical |
| -7.10% | -15.8 | (0.38 to 0.53) | (from 68 fewer to 92 fewer) | |||||||||
| HF hospitalization | ||||||||||||
| 2 | randomized trials | not serious | seriousb | not serious | not serious | none | 741/2890 | 763/2616 | OR 0.89 | 23 fewer per 1000 (from 46 fewer to 0 more) |
⊕⊕⊕ moderate |
critical |
| -25.60% | -29.20% | (0.79 to 1) | ||||||||||
| All-cause death | ||||||||||||
| 4 | randomized trials | not serious | not serious | not serious | serious | none | 556/3507 | 547/2968 | OR 0.96 | 6 fewer per 1000 | ⊕⊕⊕ moderate |
critical |
| -15.90% | -18.40% | (0.84 to 1.1) | (from 25 fewer to 15 more) | |||||||||
Note: aNo serious risk of bias; bThe results of the two studies differ significantly; cAcross equivalent lines; CI: Confidence Interval; OR: Odds Ratio; sGC: soluble Guanylate Cyclase stimulators; CV death: Cardiovascular Death; HHF: Hospitalization for Heart Failure; ⊕⊕⊕: moderate quality of the evidence; ⊕ very low quality of the evidence.
Table 2: GRADE (Grading of Recommendations, Assessment, Development and Evaluation) analysis: Quality assessment of the primary endpoint.
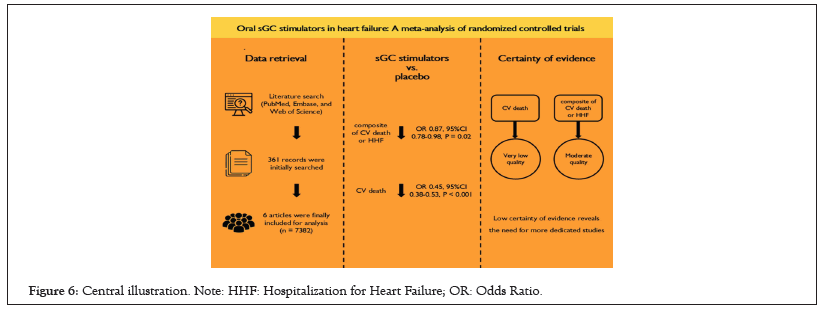
Figure 6: Central illustration. Note: HHF: Hospitalization for Heart Failure; OR: Odds Ratio.
A lack of consistency of the incidences of efficacy outcomes including composite of CV death or death or HF hospitalization, HF hospitalization, CV death of HFrEF patients, all-cause death of HFrEF patients between the two included studies downgraded the level of certainty of evidence. For safety outcomes, we downgraded the certainty of evidence to moderate due to imprecision. The certainty of evidence is low for all-cause death of HFpEF patients and dizziness, and very low for CV death. The main reasons for downgrading levels were inconsistency and imprecision (Tables 3 and 4).
| Quality assessment | No of patients | Effect | Quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | sGC | placebo | Relative | Absolute | |
| (Treatment) | (control) | (95% CI) | (95% CI) | ||||||||
| Any Adverse event | |||||||||||
| 5 | Randomised trials |
Not seriousa | not seriousb | not serious | seriousc | none | 3080/4024 | 2408/3052 | OR 0.97 | 5 fewer per 1000 | ⊕⊕⊕ moderate |
| -76.50% | -78.90% | (0.86 to1.09) | (from 26 fewer to 14 more) | ||||||||
| Any serious adverse event | |||||||||||
| 5 | Randomised trials |
Not serious | not serious | not serious | serious | none | 1148/3762 | 993/3052 | OR 0.92 | 18 fewer per 1000 | ⊕⊕⊕ moderate |
| -30.50% | -32.50% | (0.83 to1.03) | (from 39 fewer to 7 more) | ||||||||
| Any study drug-related adverse event | |||||||||||
| 4 | Randomised trials |
Not serious | not serious | not serious | not serious | none | 182/1505 | 42/567 | OR 1.44 | 29 more per 1000 | ⊕⊕⊕⊕ high |
| -12.10% | -7.40% | (1to 2.08) | (from 0 more to 69 more) | ||||||||
| Study drug–related serious adverse event | |||||||||||
| 3 | Randomised trials |
Not serious | not serious | not serious | serious | none | 20/1414 | 8/447 | OR 0.76 | 4 fewer per 1000 | ⊕⊕⊕ moderate |
| -1.40% | -1.80% | (0.33to 1.74) | (from 12 fewer to 13 more) | ||||||||
Note: CI: Confidence Interval; OR: Odds Ratio; sGC: soluble Guanylate Cyclase stimulators; alow risk of bias would indicates-no serious limitations; bI2 ≤ 50%; ccrossing the equivalence line; d50%<I2<75%. ⊕⊕⊕⊕: High quality of the evidence; ⊕⊕⊕: Moderate quality of the evidence
Table 3: GRADE (Grading of Recommendations, Assessment, Development and Evaluation) analysis: quality assessment of safety outcomes.
| Quality assessment | No of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | sGC | placebo | Relative | Absolute | ||
| (Treatment) | (control) | (95% CI) | (95% CI) | |||||||||
| CV death(HFrEF) | ||||||||||||
| 2 | randomized trials | not seriousa | seriousb | not serious | not serious | none | 221/2890 | 447/2586 | OR 0.42 | 95 fewer per 1000 | ⊕⊕⊕ moderate |
critical |
| -7.60% | -17.30% | (0.35 to 0.5) | (from 78 fewer to 105 fewer) | |||||||||
| All-cause death(HFrEF) | ||||||||||||
| 2 | randomized trials | not serious | serious | not serious | not serious | none | 530/2890 | 540/2616 | OR 0.94 | 10 fewer per 1000 | ⊕⊕⊕ moderate |
critical |
| -18.30% | -20.60% | (0.82 to 1.08) | (from 31 fewer to 13 fewer) | |||||||||
| All-cause death(HFpEF) | ||||||||||||
| 2 | randomized trials | seriousa,c | not serious | not serious | seriousd | none | 26/617 | 7/352 | OR 1.88 | 17fewer per 1000 | ⊕⊕ low |
critical |
| -4.20% | -2% | (0.83 to 4.28) | (from 3 fewer to 60 more) | |||||||||
Note: CI: Confidence Interval; OR: Odds Ratio; sGC: soluble Guanylate Cyclase stimulators; CV death: Cardiovascular Death; HFrEF: Heart Failure with Reduced Ejection Fraction; HFpEF: Heart Failure with Preserved Ejection Fraction; aIncomplete accounting of patients and outcome events; bThe results of the two studies differ significantly; cLack of blinding; d: Across equivalent lines; ⊕⊕⊕: Moderate quality of the evidence; ⊕⊕: Low quality of the evidence.
Table 4: GRADE (Grading of Recommendations, Assessment, Development and Evaluation) analysis: quality assessment of the Efficacy outcomes for HFpEF patients or HFrEF patients.
This meta-analysis investigated the effect of oral sGC stimulator in patients with HF and a total of 6 RCTs with 7382 patients. In the overall HF cohort, oral sGC stimulators reduced the risk of a composite of cardiovascular death or hospitalization for HF. For patients with HFrEF, oral sGC stimulators also significantly reduced the risk of CV death. No increased risks of safety outcomes were found in patients administered with oral sGC stimulators. However, it should be noted that only four studies investigated “hard” endpoints, while the remaining two studies reported surrogate endpoints. Moreover, the study sample size was limited, with various study endpoints and different follow-up durations. The certainty of evidence was also summarized, and no high level of certainty of evidence was found (Central illustration).
Previous research by Qi Li et al., showed that oral sGC stimulators, vericiguat and riociguat, reduce the occurrence of hospitalization due to heart failure or cardiovascular death, demonstrating good tolerability and safety [18]. Our study also found that sGC significantly reduces the composite of CV death or HHF and the CV death in patients with HF, a result that has been validated in HFrEF. Nonetheless, sGC did not achieve statistical significance for other efficacy endpoints, even after conducting sensitivity analysis [19].
The sGC is a primary receptor in the NO-sGC-cyclic GMP (cGMP) pathway. The main function of sGC stimulators is to enhance the affinity between NO and sGC, activate a series of signaling pathways, and increase cGMP levels. The increase in cGMP is associated with vasodilation, anti-fibrosis, and anti-inflammatory effects, and is beneficial for improving cardiac function and anti-remodeling effects [20,21]. Previous studies have also indicated that sGC stimulators can reduce mortality rates and improve the quality of life in patients with heart failure [22,23]. The 2021 ESC HF guidelines recommends vericiguat in the treatment of HFrEF, while the 2022 AHA/ACC/HFSA heart failure guidelines recommend the combined use of vericiguat for HF management [24]. Although sGC stimulators may play a novel role of HF treatment, our meta-analysis found that large-scale, high-level RCTs are still limited. Moreover, the certainty of evidence for the primary outcome is not high. Therefore, due to the limited number of high-quality studies and significant inter-study heterogeneity, some outcomes in this study lack consistency and precision. The results of our meta-analysis arise the necessity of more high-quality studies to provide more solid evidences supporting sGC stimulators for HF treatment.
Several limitations were considered in this study. Firstly, several potential confounders may introduce bias, including the types of sGC stimulators used, the duration of follow-up, and treatment dosages. Secondly, the limited number of studies and different study endpoints from included RCTs may increase inter-study heterogeneity. Finally, the certainty of evidence indicates that most outcomes were not solid enough, highlighting the urgent need for large-scale studies.
Currently, large-scale RCTs with enough statistical power is limited on the issue of efficacy of sGC stimulators on HF prognosis. In this meta-analysis, oral sGC stimulators may reduce the risk of CV death or HHF for HF patients, especially for HFrEF. However, the certainty of evidence is insufficient, and more dedicated studies are needed to further determine the role of sGC stimulators in HF.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Quanyu Zhang and Qiang Ren. The first draft of the manuscript was written by Qiang Ren and Sicong Ma commented on previous versions of the manuscript. All authors read and approved the final manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ren Q, Zang Q, Ma S (2024). Oral Soluble Guanylate Cyclase Stimulators in Heart Failure: A Meta-Analysis of Randomized Controlled Trials. Angiol Open Access. 12:529.
Received: 13-Nov-2024, Manuscript No. AOA-24-35148; Editor assigned: 15-Nov-2024, Pre QC No. AOA-24-35148 (PQ); Reviewed: 29-Nov-2024, QC No. AOA-24-35148; Revised: 06-Dec-2024, Manuscript No. AOA-24-35148 (R); Published: 13-Dec-2024 , DOI: 10.35841/2329-9495.24.12.529
Copyright: © 2024 Ren Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.