Research Article - (2023)Volume 9, Issue 2
Organic Dots-PVA as a Platform for Wound Dressing
Amin Shiralizadeh Dezfuli1*, Hamed Afkhami2, Iman Menbari Oskouiec3 and Leili Mohammadi4Abstract
We utilize a new nanocomposite material to give a report about a new platform for wound dressing. It is worth mentioning synergistic mixing graphene quantum dots (as a type of Organic Dots (ODs)) and Polyvinyl Alcohol (PVA) are materials participating in fabrication of the nanocomposite mentioned. We brought Staphylococcus aureus as a gram-positive bacterium and Pseudomonas aeruginosa as a gram-negative bacterium into play to optimize their antibacterial activity. We evaluated Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for OD/PVA nanocomposite. Our optimizations resulted as a persistent level against Methicillin-Resistant Staphylococcus Aureus (MRSA) in higher ratios of OD. In-vitro studies and MTT assay were used for determination the optimum ratio of OD to PVA. We quantified burned wound infection models and the colony forming units CFUs in the burn wound which were in-vivo studies by a standard colony counting method. Finally, the results show the mechanism of antibacterial activity of nanocomposite described as a contact mediated oxidative stress induction.
Keywords
Organic dots; Graphene quantum dot; Poly vinyl alcohol; GQD; Wound dressing
Introduction
Bacterial infection is one of the diseases which involve a huge population of individuals, day after day [1]. A “wound infection” is raised when a colony of bacteria intrudes through a damaged skin barrier, this is among the most common of bacterial diseases [2]. Wound infections are caused mainly because of impairment of the skin barrier due to surgery, injuries, burns, and trauma [3,4]. Today, the use of antibiotics is considered as a conventional method to treat infected wounds [5]. As the statistics claim, 23,000 people die each year due to multi-resistant infections in the U.S. However, the occurrence of Multiple Drug Resistance (MDR) in bacteria was initially identified in the late 1950s [6]. Since then, MDR is considered as being a major threat to the clinical and public health worldwide [7]. As the demands for finding alternative antimicrobial agents is spreading, attentions to this field have greatly increased as well. Utilization of Nano-materials such as nanosilver, carbon nanomaterials (like carbon nanotubes and carbon quantum dots) and graphene-based materials as antibacterial agents has winded in world on account of the discovery of graphene in 2004. Since then, its remarkable properties absorbed great attention for biomedical applications such as drug delivery, cancer therapy, tissue-engineering, bio-sensing and antibacterial agents [8-11].
Carbon-Based Quantum Dots (CBQDs) containing Graphene Quantum Dots (GQDs) and Carbon Quantum Dots (CQDs) can be categorized as organic materials and named them as “organic dots (ODs)” [12].
Remarkable developments in biomedicine field were caused, due to ODs. To make it more pointed, low toxicity, fluorescence, high photo stability against photo bleaching, good solubility, and high biocompatibility perform the fascinating properties [13-17]. Also, in the role of an effective antibacterial material, ODs has been suggested recently [18-20]. It is a zero-dimensional material with sp2-sp2 carbon bonds which can act as a piece of tiny graphene. In process of the antibacterial activity of ODs mechanisms such as oxidative stress, reactive oxygen species or direct extraction of phospholipid from bacterial membranes play a role, as it was suggested [21]. VTH Pham et.al indicated that antibacterial agent action of graphene sheets, happen through the formation of pores in bacterial cell walls followed by an osmotic imbalance and cell death [22]. Sun et al recently reported that the high peroxidase-like activity, plus an antibacterial system based on H2O2 decomposition catalyzed by ODs [19]. It is noteworthy to mention that peroxidase-like activity of ODs is significantly higher than Graphene, apparently properties such low toxicity and high biocompatibility, support the state that ODs is a suitable candidate for disinfection of wounds [17,19]. Wound healing requires a special environment as it is a complex and crucial process, encouraging wound dressing which can directly contact with the wound, protects the wound bacterial infections, and accelerates the healing process [23,24]. More specifically, a proper environment is provided, in order that, the process of collagen synthesis is stimulated and it creates hypoxia which leads to an increase in angiogenesis as well as faster re-epithelialization. "Hydrogel dressing" is an obliging method for wound dressing, which comprehends hydrophilic insoluble materials that are mostly composed of the non-biological and synthetic polymers such as Polyvinylpyrrolidone (PVP), Polyethylene Oxide (PEO) and Polyvinyl Alcohol (PVA) etc. [23-27].
Since hydrogels can absorb and maintain water up to 90% and have 3-dimensional mesh-like structure, plasticity, plus they’re similar to the physiological environment, also are biocompatible, and biodegradable, it is considered that they can remarkably be employed for biomedical applications such pharmaceutical field, tissue engineering, wound dressing, and drug delivery [28-33].
“PVA hydrogel” is a type of hydrogel which is hydrophilic as it has numerous -OH groups and what’s more it can moisturize the wound environment to absorb exudates of the wound and increase the migration of keratinocyte and fibroblast proliferation [34-37]. Furthermore gas exchange, easy loading, and sustained release of drugs are options that its unique structure (interconnected pores) allows it to have [38,39]. We should report that PVA hydrogel is commonly used in wound healing, wound management, and drug delivery systems, as an appropriate option for the wound dressing PVA hydrogel is considered suitable enough due to its high biocompatibility, biodegradability, and most importantly the none-toxicity [40-42].
In consequence, in our study the antibacterial activity of ODs embedded in cross-linked PVA hydrogel was examined for the first time.
Material and Methods
Materials and instruments
We obtained Transmission Electron Microscopy (TEM) images as we employed a Model CM30 electron micro-scope (Philiphs). What’s more, for acquiration of the Field-Emission Scanning Electron Microscopy (FE-SEM) data on the gold-coated samples we utilized A Zeiss SIGMA VP system. By using a BRUKER EQUINOX 55 device the acquiration of The Fourier Transform Infrared (FT-IR) was resulted.
Synthesis of ODs
According to Bhowmick et al, For the synthesis of GQDs procedure, 5 g of Citric Acid (CA) was heated in a at 200°C in a 50 mL beaker using an oil bath [43]. While the color of liquid CA changed from colorless to yellow the formation of the GQDs was indicated. Then, 0.1 M NaOH solution was added dropwise into the resultant GQDs under constant stirring to achieve pH 7.0. The final concentration of GQD solution was adjusted at 0.4 g/ml by adding deionized water.
Preparation of OD/PVA
For preparation of PVA/OD composite, we can explain as PVA was initially dissolved in deionized water by refluxing at 70°C in order to prepare a 10% w/w PVA solution. As the second step, differing quantities of the GQD solution (0.4 g/ml) were added to the prepared PVA solution for the reason to have different dry mass ratios of OD/PVA. 20% solution of potassium phosphate was added to the OD/PVA in a mold this action eventuate the hydrogel preparation.
Biological studies
Minimum Inhibitory Concentration (MIC): A quantitative antibacterial test by the culture of Pseudomonas aeruginosa and Methicillin-Resistant Staphylococcus Aureus (MRSA) in Luria-Bertani (LB) agar plate at 37°C for 24 hours was done as MIC (Minimum inhibitory concentration) experiment. To continue, we transferred 2-5 colonies into 2 ml of saline solution with a sterile loop. The optical density was adjusted from 620 to 0.8. 3 ml was the final volume of the sample concentrations for negative and positive controls. To give additional information we can report that various ratios of OD to composite were prepared and added to the solutions in order have OD/PVA composites. The solutions mentioned in last part were containing equal amounts of saline solution, LB broth and the bacteria. While, the solutions were prepared, we incubated them, at 37°C for 24 hours. The MBC test was carried out with re-inoculating 10 μl of each culture medium from the test tube on nutrient agar plates in order to increase the confirmation of the antibacterial efficiency.
In furtherance of confirmation of the antibacterial efficiency of OD/PVA, the Nanodrop (Thermo Scientific, NanoDrop, OneC Spectrophotometer) was used in order to measure The optical density of the samples is 620 nm. The cell viability of both P. aeruginosa and MRSA was calculated by the:

MTT Assay: MTT test evaluated the cytotoxicity effect of OD/PVA composites on MCF-7 cell line. As we know the ratios of OD/PVA indicated the antibacterial activity in antibacterial tests, besides the ratios of OD/PVA was examined. By using a regular method, MCF-7 cells were cultured in DMEM F12 medium containing 10% FBS and after three subcultures, the cells were seeded at the density of 1 × 104 cells/well in a 96-well plate for a 24 hour duration. Our observation showed that OD/PVAs were dispersed in PBS at pH=7.4 to make the sample groups and were then added into the wells. Additionally all the experiments were performed in triplicate and were repeated at least twice as some wells were left untreated as negative controls. It’s valuable to mention that MTT assay was performed 36 hours after the treatment of MCF-7 cell lines with OD/PVA composites by replacing the medium with 100 µμl of RPMI1640 and 10 μl of 12 mM MTT solution. The medium was removed and 100 μl of DMSO was added to each well to dissolve the insoluble formazan product into a colored solution, this action was done after the incubation of the plate for additional 4 hours at 37°C. At all last, the absorbance was determined at 570 nm using ELISA reader. The percentage of cell viability was obtained by using the following equation:
Cell viability (%)=(Absorbance of treated cells )/(Absorbance of untreated cells ) × 100

In-vivoAntibacterial evaluation: We report that mouse burn models were built in order to evaluate the potential effect of Polyvinyl Alcohol (OD-PVA) nanocomposite in-vivo. The animals were anesthetized with the ketamine/xylazine (50-90 mg/kg, i.p.), and electric clippers were used to hair removal prior to the burn. As the next step, the third-degree burn wounds were created by pressing the custom-made mold with a 1.5 cm × 1.5 cm window on the back of the mice after exposing to a gas flame for 9 secs. In order to replace the fluid, saline solution and glucose were given to the mice. Later, the overnight cultured (at 37°C) P. aeruginosa PAO1 strain was administered beneath the burn wound.
In subsequent, both groups of 6 male mice were divided into Saline and OD-PVA Nano-composite groups. In order to increase the confirmation of the antibacterial activity of OD-PVA against P. aeruginosa and MRSA in-vivo the CFUs in the burn wound were quantified, this action was done by a standard colony counting method. Concisely, all mice were sacrificed after 7 days and the harvested tissues were homogenized in 1 cc of Phosphate Buffered Saline (PBS) and 9 cc of PBS was subsequently added to prepare serial dilutions from 10-1 to 10-8. On Nutrient agar medium, Aliquots of diluted homogenized burn wound tissues were placed and then, the colonies were counted.
Results and Discussion
FT-IR: In this study for evaluating the chemical structure of the ODs synthesized by incomplete pyrolysis of citric acid (Figure 1a), FT-IR was used as a conventional method. C=C bonds were indicated by the skeletal vibrational of the none-oxidized aromatic group which was observed at 1650 cm-1. The results were observed at1022 cm-1, 1203 cm-1 and about 1720 cm-1 were allocated correlatively to C-O-C stretching, C-OH stretching and C=O stretching mode of vibration.
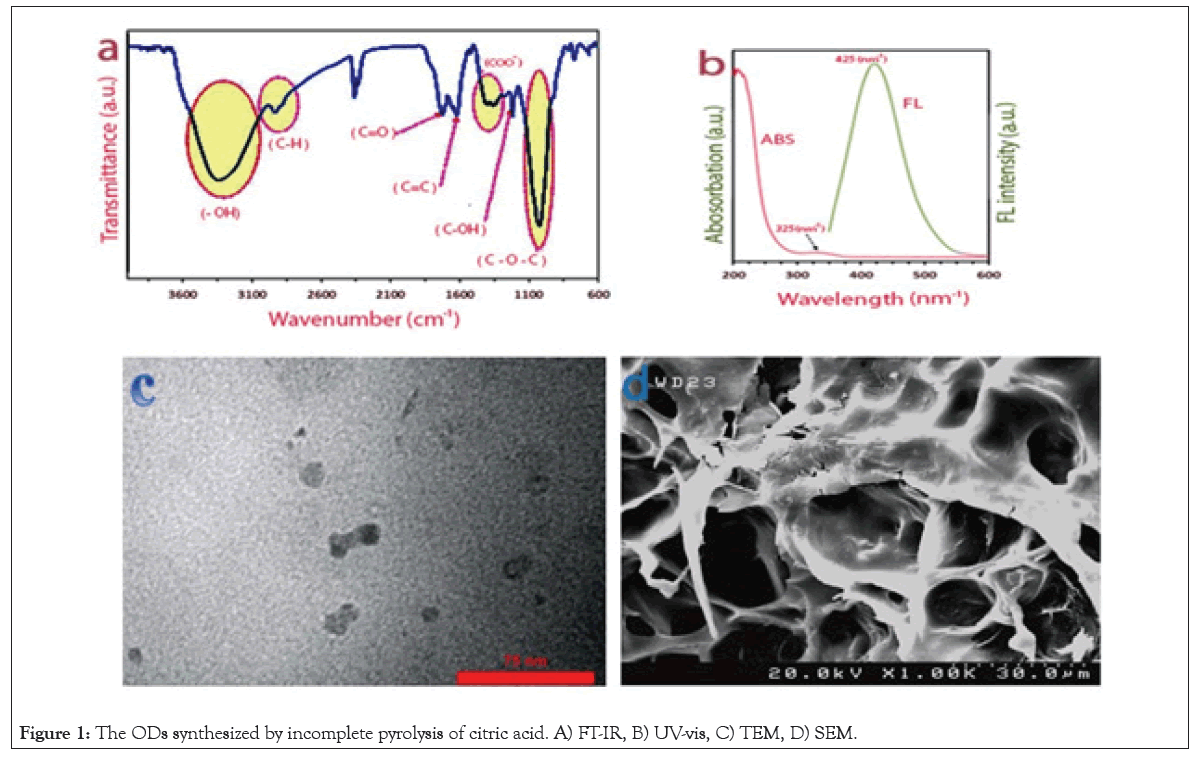
Figure 1: The ODs synthesized by incomplete pyrolysis of citric acid. A) FT-IR, B) UV-vis, C) TEM, D) SEM.
The incomplete carbonization of CA during pyrolysis is the reason for the absorption of the C-H stretching vibration at the range of 2600-3100 cm-1 shown by The OD. the existence of -COOH functional groups in the ODs was indicated by reporting that The broad band peak of -OH and the carboxyl group are at the range of 3100-3600 cm-1 and 1100-1600 cm-1.
UV/Vis and PL spectroscopies: The results of UV/Vis absorption and PL spectra of the ODs are shown in Figure 1b. On account of the π→π* transition of aromatic sp2 domains a typical absorption peak at 230 nm was observed. There are relations between thee n→π* transition of carbonyl groups and a broad band at 332 nm which was observed. The PL property of the OD was evaluated under the excitation by 360 nm UV light. However, the emission wavelength of ODs showed the size dependent properties, it was probably excitation-independent and were given the blue luminescence and broad peak at 425 nm in this trial. We can claim that these Organic dots are ODs type II [12].
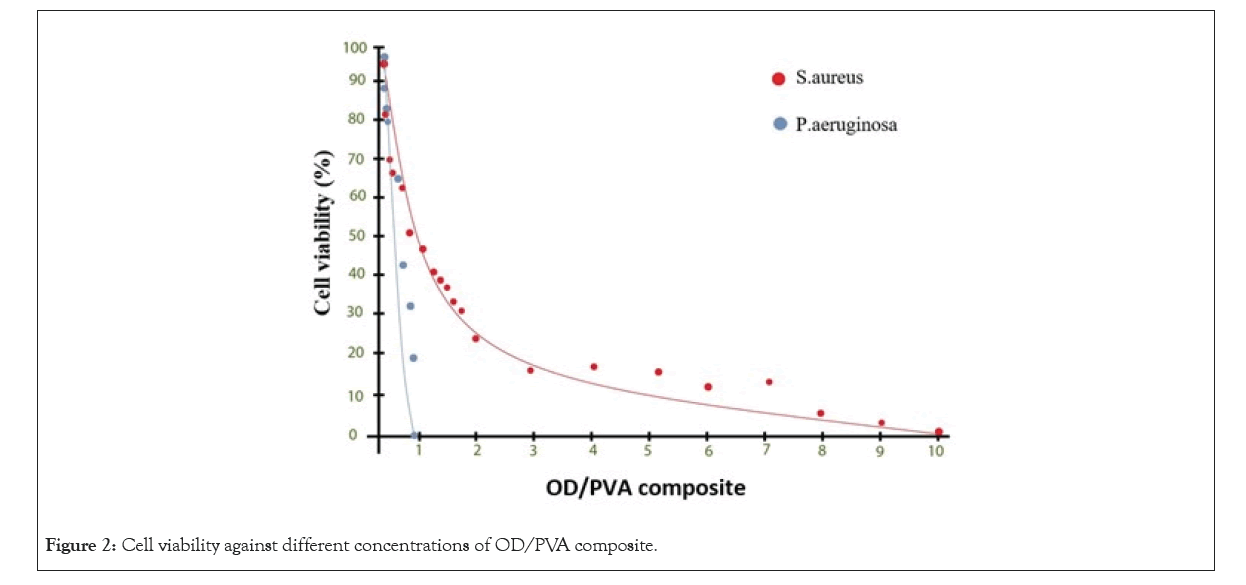
Figure 2: Cell viability against different concentrations of OD/PVA composite.
Transmission Electron Microscopy (TEM) and SEM: Transmission Electron Microscopy (TEM) image of ODs was used for confirmation of the size distribution of ODs (Figure 1c, 1d). This was characterized by Dynamic Light Scattering (DLS) which showed a relatively identical size distribution of 5-20 nm diameters.
Effects of OD/PVA on the growth of microbial strain
MIC:There was a commensurate connection between the differences in concentrations of OD/PVA against P. aeruginosa and MRSA bacteria, as the results of the antibacterial test shown in Figure 2. In MRSA the growth of the bacteria in 0.04% of OD/PVA was indicated when the strong turbidity was appeared.As an explanation of the case which was mentioned, it was suggested that there was a logarithmic decline in the cell viability in case of increasing the amount of OD in the OD/PVA composite. When the OD reached 0.3% there was a great decrease in the turbidity while a clear solution was observed at 10% of OD in the OD/PVA composite. In this experiment as positive control, a solution of bacteria in LB broth solution and a broth solution without bacterial treatment was used as a negative control. In P. aeruginosa, pyocyanin and pyoverdine showed the blue-green characteristic color of the culture medium which is not surprising in the positive control. The growth of bacteria was confirmed by the samples containing OD/PVA with OD, due to the amounts ranging from 0.04% to 0.16% which showed the same color. The intersection of plot with the horizontal axis demonstrated that MIC values for P. aeruginosaand MRSA were 0.6% and 8%, respectively (Figure 2).
While the cell viability ofMRSAhave a logarithmic behavior from the Figure 2, results indicates the percent of OD increased the cell viability of P. aeruginosashowed a linear decrease. It was observed that both the bacteria strains showed similar reduction rate in the cell population up to 50%, although after that the reduction rate of MRSA cell population was much slower in comparison with P. aeruginosa. these observations hinted that MRSA was probably persistent to high ratios of OD and the bactericidal rate was much slower in comparison with the bactericidal properties at lower ratios. There was a strong correlation between the peptidoglycan bacterial cell wall in MRSA and different structural properties of Gram-negative and Gram-positive bacteria due to the obsevations.
MBC:The MBC result of this study is shown in Figure 3. In this experiment with an increase in OD concentration the numerous colonies in the bacterial culture of positive control were reduced. This action hinted that the OD had a certain antibacterial activity against P. aeruginosa and S. aureus which caused the improvement in the gradual increase in the amount of OD. Additionally the huge difference in MBC concentration of P. aeruginosa against S. aureusindicated the better efficiency of the antibacterial activity of OD/PVA againstP. aeruginosa. Furhermore, the number of colonies in MBC test plates containing 0.16% and 0.2% OD/PVA suggested that the number of colonies in 0.16% and 0.2% OD/PVA is similar, although the number of colonies significiantly reduced at the same OD/PVA concentrations in P. aeruginosa culture plates, in addition it was consistent with the MIC test as well (Figure 3).
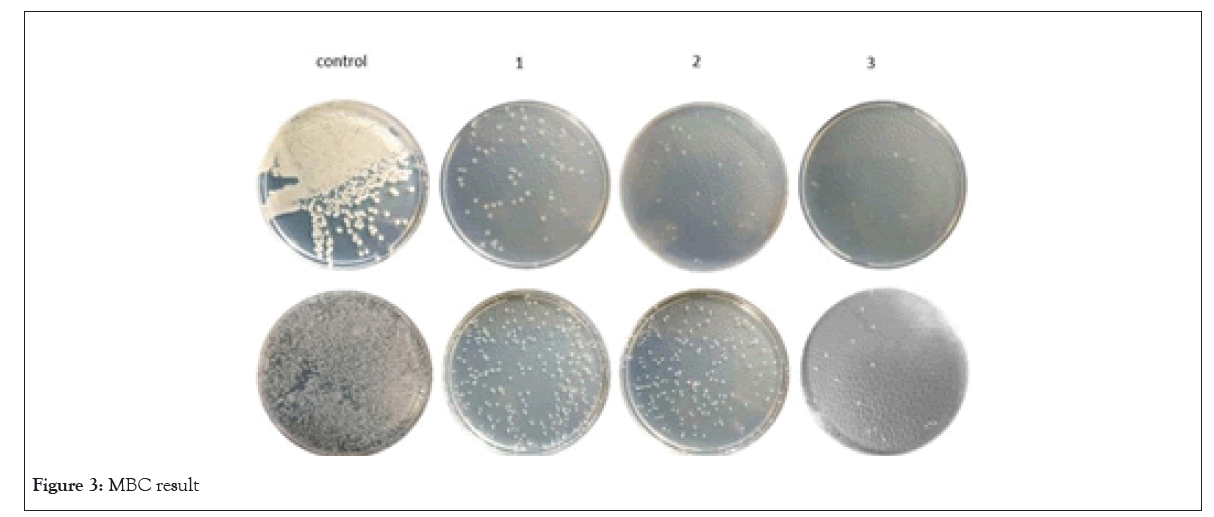
Figure 3: MBC result
MTT assay: In this experiment we’re reporting that for determination of the biocompatibility of the composites the cytotoxicity of OD/PVA was evaluated using MTT assay. The test group was treated by the ratios alike the ones in MIC and MBC. Due to the results shown in Figure 4, there is a linear correlation between the percent of OD and the toxicity, in such a way that the toxicity enhanced by increasing the percent of OD in the OD/PVA composite. The cell viability relative to the control was about 37.78% at 10% OD and was more than 80% at the OD concentrations lower than 3%. We can infer that it could be applicable for antibacterial purposes as the calculated trendline, more than 90% of the cells were expected to remain viable in OD/PVA ratios more than 2% and OD/PVA was minimally toxic. The ratios of OD which led to eliminate more than 50% of the bacteria were determined to be safe in terms of toxicity (0.30% and 0.38 % of OD in the composite, against two gram-negative and positive bacteria strains, respectively). However, in case of elimination of all the bacteria OD/PVA wasn’t successful enough for MRSA due to its requirement for more safeness ratio. The observations showed that using OD/PVA is not suitable and even toxic for both eukaryotic and prokaryotic cells so using the optimal ratio of this material to achieve the best antimicrobial activity is considerably important. OD/PVA can be used as an effective antibacterial agent in-vitro or in-vivo for external use, because of the less toxicity of OD/PVA against the eukaryotic cells unlike high bactericidal activity in low ratios, especially against gram-negative bacteria like P. aeruginosa (Figure 4).
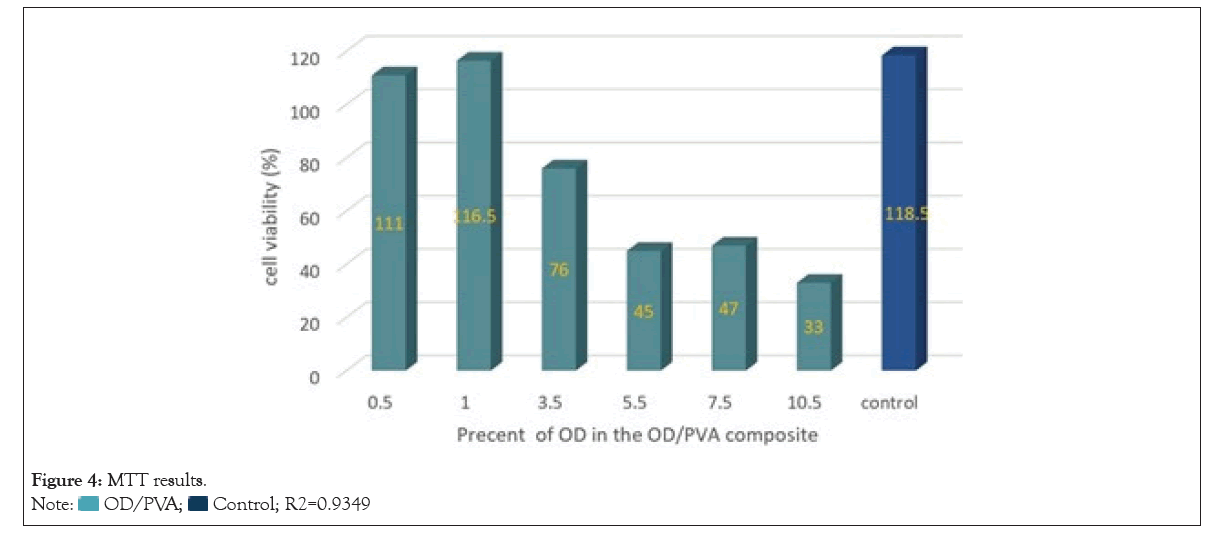
Figure 4: MTT results.

y=-7.7742x+105.54
In-vivoresults:In this study we can report that the number of the bacteria colonies on agar plate at serial dilution of 10-8 was about 35 and 57 for P. aueroginosa and MRSA, respectively after the treatment of the mice with burned wound infection with OD-PVA nanocomposite, although more than 105 bacteria colonies were observed in positive controls.
The observations resulted in the way indicated that after the treatment of the burned wound infection mice with OD-PVA nanocomposite the number of the bacteria (gram-positive and negative) reduced significantly, it’s good to mention that there was an agreement between the in-vivo results and in-vitro results (antibacterial effect of OD-PVA on gram-the negative bacteria was more than the gram-positive bacteria).
In point of fact, the mechanism causing the antimicrobial activity of nanomaterials is still vague in many areas and fully understanding it is still remained a challenge. However in recent studies on graphene based nanomaterials, the induction of the oxidative stress has been proposed as the main reason for their antimicrobial activity giving rise to the disruption of the bacterial cell membrane integrity [44,45]. Additionally another reason for the antimicrobial activity of graphene can be announced as physical penetration of graphene through the cell membrane that occurs due to its sharp edges [19,45,46]. In recent studies, parameters such as graphene size, physical contact, the strain of the bacteria, and the variety of sizes, shapes, and the structural defects dependent on the graphene source are collated to the antibacterial activity of graphene [20]. What’s more, in studies the association of the attachment and contact of the bacteria and the antibacterial activity has been shown [20].
We should report that in our study OD, as a Nanometric graphene material, in composition with PVA indicated an effective antibacterial activity against both P. aeruginosa and S. aureus. The composite which was mentioned, acted more effective against P. aeruginosa as a gram-negative and an antibacterial activity against S. aureus in higher ratios of OD was observed from it. However there is a surface limitation to make an effective contact with planar graphene which has a zero Gaussian curvature as Since S.aureus is a round-shaped gram-positive bacteria with a size smaller than P. aeruginosa.
Altogether, effective attachment and contact to the bacteria followed by the direct oxidation and the ability to disrupt the cell membrane are suggested to be related to the mechanism of the antibacterial activity of OD. This can also describe the reason for why the smaller round shape gram-positive bacteria show less sensitivity to the OD and a persistent level in higher ratios of OD. We assume that the attachment of a single OD to the surface of the bacteria makes the subsequent attachments limited for other ODs. What’s more the induction of the oxidative stress will not be effective to disrupt the integrity of the bacterial cell, especially in gram-positive bacteria, if the connection of OD is not optimal.
Conclusion
To summarize, the preparation of a novel OD/PVA hydrogel was by a simple procedure and the antibacterial activity of this hydrogel which investigated on burn wound infection mice caused by MRSA and P. aeruginosa. No cell toxicity was observed in the MIC value while significant antimicrobial activity was observed against P. aeruginosa, particularly. However, significant level of persistency in higher concentrations of OD was showed by MRSA strain, this occurrence indicated that the OD/PVA hydrogel can be used against P. aeruginosa infectious disease and wound dressing applications due to its potential.
References
- Rizzello L, Pompa PP. Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chem Soc Rev. 2014;43(5):1501-1518.
- Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063-1072.
- Nichols RL. Surgical wound infection. Am J Med. 1991;91(3):S54-S64.
- Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19(2):403-434.
- Fernández J, Acevedo J, Castro M, Garcia O, Rodriguez de LC, Roca D, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatol. 2012;55(5):1551-1561.
- CfD, C. Prevention: Antibiotic resistance threats in the United States, 2013.
- Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12):S122-S129.
- Hu W, Peng C, Luo W, Lv M, Li X, Li D, et al. Graphene-based antibacterial paper. ACS nano. 2010;4(7):4317-4323.
- Sotiriou GA, Pratsinis SE. Antibacterial activity of nanosilver ions and particles. Environ Sci Techno. 2010;44(14):5649-5654.
- Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, et al. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS nano. 2011;5(9):6971-6780.
- Kim JD, Yun H, Kim GC, Lee CW, Choi HC. Antibacterial activity and reusability of CNT-Ag and GO-Ag nanocomposites. Appl Surf Sci. 2013;283:227-233.
- Dezfuli AS, Kohan E, Fateh ST, Alimirzaei N, Arzaghi H, Hamblin MR. Organic dots (O-dots) for theranostic applications: preparation and surface engineering. RSC Adv. 2021;11(4):2253-2291.
- Shen J, Zhu Y, Yang X, Li C. Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem Comm. 2012;48(31):3686-3699.
- Zhang Z, Zhang J, Chen N, Qu L. Graphene quantum dots: an emerging material for energy-related applications and beyond. Energy Environ Sci. 2012;5(10):8869-8890.
- Zhu S, Tang S, Zhang J, Yang B. Control the size and surface chemistry of graphene for the rising fluorescent materials. Chem Comm. 2012;48(38):4527-4539.
- Li L, Wu G, Yang G, Peng J, Zhao J, Zhu JJ. Focusing on luminescent graphene quantum dots: current status and future perspectives. Nanoscale. 2013;5(10):4015-4039.
- Chong Y, Ma Y, Shen H, Tu X, Zhou X, Xu J, et al. The in vitro and in vivo toxicity of graphene quantum dots. Biomater. 2014;35(19):5041-5048.
- Ristic BZ, Milenkovic MM, Dakic IR, Todorovic MBM, Milosavljevic MS, Budimir MD, et al. Photodynamic antibacterial effect of graphene quantum dots. Biomater. 2014;35(15):4428-4435.
- Sun H, Gao N, Dong K, Ren J, Qu X. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano. 2014;8(6):6202-6210.
- Hui L, Huang J, Chen G, Zhu Y, Yang L. Antibacterial property of graphene quantum dots (both source material and bacterial shape matter). ACS Appl Mater Interfaces. 2016;8(1):20-25.
- Amani H, Habibey R, Hajmiresmail SJ, Latifi S, Pazoki-Toroudi H, Akhavan O. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries. J Mater Chem B. 2017;5(48):9452-9476.
- Pham VT, Truong VK, Quinn MD, Notley SM, Guo Y, Baulin VA, et al. Graphene induces formation of pores that kill spherical and rod-shaped bacteria. ACS Nano. 2015;9(8):8458-8467.
- Razzak MT, Darwis D. Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat Phys Chem. 2001;62(1):107-113.
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomater. 2003:24(24):4337-4351.
Author Info
Amin Shiralizadeh Dezfuli1*, Hamed Afkhami2, Iman Menbari Oskouiec3 and Leili Mohammadi42Department of Medical Microbiology, Shahed University, Tehran, Iran
3School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
4Infectious Diseases and Tropical Medicine Research Center, Zahedan University of Medical Sciences, Zahedan, India
Citation: ezfuli AS, Afkhami H, Oskouiec IM, Mohammadi L (2023) Organic Dots-PVA as a Platform for Wound Dressing. Appli Microbiol Open Access. 9.254.
Received: 28-Feb-2023, Manuscript No. AMOA-23-21985; Editor assigned: 02-Mar-2023, Pre QC No. AMOA-23-21985(PQ); Reviewed: 20-Mar-2023, QC No. AMOA-23-21985; Revised: 28-Mar-2023, Manuscript No. AMOA-23-21985(R); Published: 05-Apr-2023 , DOI: 10.35248/2471-9315.23.9.254
Copyright: © 2023 Dezfuli AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.