Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2010) Volume 2, Issue 1
The objectives of this longitudinal clinical outcomes study were to: (1) compare rates and types of adverse events (AEs), adherence scores, and quality-of-life (QoL) scores in patients using ritonavir-boosted protease inhibitor (PI) regimens versus nonnucleoside reverse transcriptase inhibitor (NNRTI) regimens; and (2) determine the relationship between speed of initial HIV-1 RNA reduction after initiating highly active antiretroviral therapy and time to resistance development (or treatment failure). A total of 198 ritonavirboosted PI- and 271 NNRTI-initiating patients were evaluated. The time to achieve suppressed HIV-1 RNA (<50 copies/ mL) ranged from 4.5 to 6 months. Although the time to discontinuation of initial therapy between the groups was similar, a shorter time to viral suppression was associated with a longer duration of effective therapy. Approximately 75% of patients achieving undetectable HIV-1 RNA maintained suppressed HIV-1 RNA over a 2-year follow-up period, although those patients receiving an NNRTI-based regimen were more likely to maintain suppressed HIV-1 RNA than those receiving a PI-based regimen (log-rank test; p=0.05). No significant differences in AEs, adherence, or QoL were reported. As newer classes of drugs become increasingly used in clinical practice, it will be important to determine whether improved outcomes will result.
<Keywords: Adverse events; Adherence; Boosted regimens; Nonnucleoside reverse transcriptase inhibitors; Quality of life; Resistance
AE: Adverse Events; NNRTI: Nonnucleoside Reverse Transcriptase Inhibitor; PI: Protease Inhibitor; QoL: Quality Sof Life
Since its introduction in 1996, potent combination antiretroviral (ARV) therapy has changed the course of HIV disease from an invariably fatal illness to a chronic but manageable one. Recommended as preferred ARV regimens in treatment guidelines (Hammer et al., 2008; US Department of Health and Human Services, 2009), protease inhibitor (PI)-, integrase inhibitors, or nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens with nucleoside reverse transcriptase inhibitors (NRTIs) are associated with potent and durable viral suppression (US Department of Health and Human Services, 2009; Lichterfeld et al., 2003; Wood et al., 2007). In some studies with PI or NNRTI-based regimens, the time to HIV-1 RNA reduction and suppression after the initiation of a PI or NNRTI regimen has been associated with subsequent durability of HIV- 1 RNA suppression, as well as improvement in other aspects of HIV disease progression (i.e., increased CD4 cell count and reduced incidence of opportunistic infections) (Powderly, 2002; Carr et al., 1998; Flint et al., 2009; Sax and Kumar, 2004; Crane et al., 2006; Fellay et al., 2001; Mary-Krause et al., 2003).
Adverse events (AEs) are common in patients receiving ARV therapy. One study showed that more than two thirds of ARVtreated patients experienced one or more clinical or laboratory AEs (Fellay et al., 2001). Treatment-limiting AEs include gastrointestinal (GI) intolerance, abnormalities in liver function, and adverse changes in lipid profiles (Powderly, 2002; Fellay et al., 2001; Shikuma et al., 2007). In particular, PIs have been associated with a cascade of cardiometabolic effects, including lipodystrophy, insulin resistance, dyslipidemia, elevated blood pressure (Powderly, 2002; Carr et al., 1998; Flint et al., 2009; Crane et al., 2006), and myocardial infarction (Mary-Krause et al., 2003; Lundgren et al., 2009). The use of ritonavir to increase the serum levels of most of the PIs results in more rapid virologic suppression (Lichterfeld et al., 2003; Wood et al., 2007), but at the expense of increasing the AE rate even further as ritonavir has been independently associated with diarrhea, hyperbilirubinemia, and elevations in cholesterol and triglycerides (Fellay et al., 2001; Aberg, 2009). The NNRTIs have been associated with their own constellation of AEs, including central nervous system disturbances (i.e., efavirenz) (Fellay et al., 2001), liver toxicity (Fellay et al., 2001; Rivero et al., 2007; Kontorinis and Dieterich, 2003; Abrescia et al., 2005), and lipid abnormalities (Shikuma et al., 2007).
Adverse events have been shown to compromise quality of life (QoL) and interfere with adherence to ARV regimens (Powderly, 2002; Trotta et al., 2002; Ammassari et al., 2001). The most serious consequence of nonadherence for this group of drugs is the emergence of resistance and, ultimately, treatment failure (Parienti et al., 2004; Sethi et al., 2003). In a univariate analysis of patients receiving highly active antiretroviral therapy (HAART), side effects were significantly associated with nonadherence (Ammassari et al., 2001). Parienti et al. demonstrated that the emergence of cross-resistance to NNRTIs increases in patients with low adherence (Parienti et al., 2004), while other studies have shown that high adherence is required to avoid the emergence of resistance and subsequent virologic failure (Sethi et al., 2003; Gross et al., 2006). Therapy for HIV disease is lifelong; yet in a large European observational longitudinal study, 21% of treatment-experienced patients and 11% of treatment-naïve patients experienced triple-class virologic failure 6 years after starting ARV therapy (p<0.0001; log-rank test) (Mocroft et al., 2004).
The current study was designed to utilize the resources of the Johns Hopkins HIV Clinical Cohort (JHHCC) to (1) compare rates and different types of AEs, adherence scores, QoL scores, and changes in lipid parameters in patients using ritonavirboosted PI regimens versus NNRTI regimens; and (2) determine the relationship between speed of initial HIV-1 RNA reduction after initiating HAART and time to resistance development (or treatment failure). Such an analysis from a large clinical practice presents a baseline with which to compare newer drug classes (e.g., integrase inhibitors, chemokine receptor antagonists, second-generation PIs, and NNRTIs) as utilization of the newer drugs increases.
This study is based on longitudinal data obtained from the JHHCC, a clinic-based cohort that encompasses urban, suburban, and rural locations throughout Maryland. Enrollment into the JHHCC coincides with enrollment into care at Johns Hopkins Hospital and at affiliated clinics in Maryland. The demographic and clinical characteristics of the JHHCC are similar to those of the overall Maryland HIV/AIDS epidemic.
Data collection
Demographic, clinical, and therapeutic data are collected longitudinally by: (1) clinical record abstraction and automation; (2) electronic linkage and acquisition of patient data; (3) patient interview by computer-assisted questionnaire; and (4) specimen (blood) collection. Institutional Review Board (IRB)-approved written informed consent was adhered to and consent forms were administered to patients at the time of their enrollment into HIV care. To date, only 1.2% of all patients enrolled into clinical care have refused study participation. Focus was limited to HIV-infected persons receiving longitudinal medical care. Data collected included demographics, medication use, laboratory values, clinical outcomes, and information about QoL and adherence obtained by audio computer-assisted self-interview.
Demographics
Data on age, gender, self-reported race/ethnicity, and HIV transmission risk group were collected.
Pharmacy/medications
All ARV drugs and other medications were recorded. Data collection included start and stop dates, dose, and duration. Additionally, for selected ARVs, AEs were categorized by specific type, interruptions in therapy, and reasons for dosing change.
Laboratory values and clinical outcomes
More than 95% of our laboratory data (including all ambulatory and inpatient laboratory data) were collected electronically through a direct link to the Johns Hopkins electronic laboratory databases (chemistry, hematology, immunology, virology, histopathology, microbiology). Data were also obtained electronically from off-site laboratories used by Johns Hopkins, specifically, Quest Diagnostics and LabCorp, which provide 90% of off-site laboratory utilization. For this analysis, laboratory data included CD4 cell count, HIV-1 RNA, liver enzymes (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TGs). Only data on fasting lipids were reported.
Audio computer-assisted self-interview
Audio computer-assisted self-interview (A-CASI) was used to collect information including adherence to ARV therapy (selfreported number of days with at least 1 missed dose over the previous 3 days) and QoL. Quality of life assessment was based on a self-reported ascending 0–100 linear analogue scale (0 = worst; 100 = best). Both an “activities of daily living” and a total QoL scale were used.
Patient sample
Antiretroviral treatment-naïve patients who first initiated treatment after January 1, 2001, on one of the following HAART regimens: (1) a non-ritonavir-boosted PI-based regimen; (2) a ritonavir-boosted PI-based regimen; or (3) an NNRTI (non-PI)- based regimen were identified. The PIs prescribed included lopinavir, fosamprenavir, amprenavir, atazanavir, and indinavir. The NNRTIs prescribed included efavirenz and nevirapine.
Statistical analysis 1: Outcomes of boosted versus nonboosted HAART regimens
Each patient was longitudinally followed from time of HAART initiation to first switch in therapy. “Switch in therapy” was defined as initially treatment-naïve patients switching from their first regimen. For patients initiated on a non-boosted NNRTIbased regimen, “switch in therapy” was defined as the patient switching to any PI-based regimen (boosted or non-boosted). Finally, patients initiated on a boosted PI-based regimen were defined as having switched therapy if they switched to a nonboosted PI-based or a non-boosted NNRTI-based regimen. Patients initiated on a boosted regimen who switched to a different boosted regimen were not considered to have switched therapies.
Using log-rank statistical analysis, Kaplan-Meier estimates of the probability of first switch among the regimen groups were computed and compared. As an extension of this analysis, Cox proportional hazards regression was used to compare the relative hazard of switch among the regimen groups. A multivariate Cox analysis was adjusted for sex, race, age, HIV transmission risk (injection drug use, men who have sex with men, heterosexual sex), and baseline HIV-1 RNA, CD4 cell count, liver enzymes, and lipid levels.
Statistical analysis 2: Speed of HIV-1 RNA reduction
Patients were eligible for this analysis if they were treatment naïve at baseline and had baseline HIV-1 RNA levels >1000 copies/mL. Patients were differentiated based on those initiating an NNRTI-based regimen or a PI-based regimen (as defined above in Patient Sample).
The time to reduce HIV-1 RNA to an undetectable level (defined as <50 copies/mL) was determined for each patient. Kaplan-Meier estimates of time to undetectable HIV-1 RNA were computed for each of the regimen groups. As an extension of this analysis, Cox proportional hazards regression was used to compare the relative hazard of HIV-1 RNA reduction among the regimen groups. A multivariate Cox analysis was adjusted for sex, race, age, HIV transmission risk, and baseline HIV-1 RNA level, CD4 cell count, liver enzymes, and lipid levels.
Additional analyses
Additional analyses included QoL and adherence, lipids, time to virologic rebound, and resistance. Mean QoL and mean ARV adherence scores acquired through self-reporting by audio-assisted self-interviewing were determined during the follow-up period. Fasting lipid values, mean total cholesterol, LDL-C, HDL-C, and TGs were determined during the follow-up period. Changes in mean values from start of therapy to switch in therapy were compared by t test. Time to virologic rebound was determined for each patient from the point of undetectable HIV-1 RNA. Rebound was defined as an HIV-1 RNA level >500 copies/ mL. Kaplan-Meier estimates were computed for each of the ARV regimen groups. Depending on whether patients initiated therapy on an NNRTI- or PI-based regimen, the number of patients acquiring genotypic NNRTI resistance or PI resistance, respectively, was determined. For purposes of this analysis, resistance was defined as the acquisition of a major NNRTI or PI mutation.
A total of 198 patients initiating ritonavir-boosted PI regimens and 271 patients initiating NNRTI regimens were studied for the initial analysis. Ritonavir-boosted PIs included lopinavir (n=114), amprenavir (n=2), fosamprenavir (n=12), indinavir (n=3), and atazanavir (n=67). NNRTIs included efavirenz (n=259) and nevirapine (n=12). (Of note, the newer PIs, tipranavir and darunavir, and the NNRTI, etravirine, were unavailable at the time of study initiation). Since January 1, 2001, only 3 patients initiated therapy with a non-boosted PI, all of whom received atazanavir. Because of this very small sample size, these patients were not studied further. Patients who initiated therapy on both a PI and an NNRTI (n=15) were also excluded from this analysis.
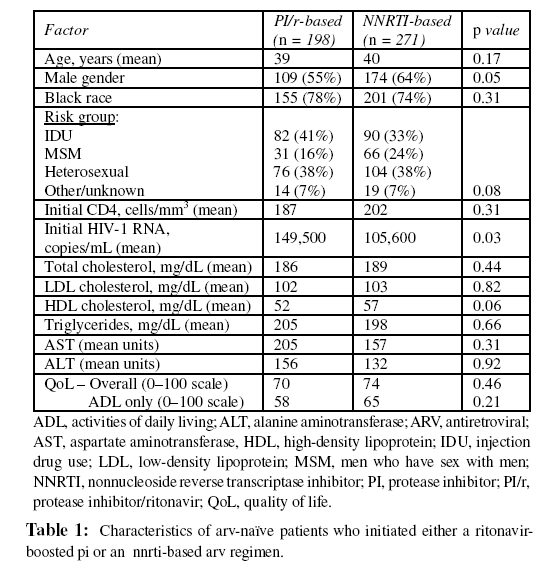
Characteristics of patients initiating either a ritonavir-boosted PI or an NNRTI are shown in Table 1. A significantly higher proportion of men than women initiated an NNRTI. Also, patients initiating a ritonavir-boosted PI had a significantly higher HIV-1 RNA. No other differences in baseline measures were observed.
Time to undetectable HIV-1 RNA
Using Kaplan-Meier methods, time from initiating therapy to first HIV-1 RNA level <50 copies/mL over the first year of therapy was examined (Figure 1). A total of 162 patients achieved a suppressed HIV-1 RNA on an NNRTI, with a median time to HIV-1 RNA <50 copies/mL of 140 days. A total of 114 patients achieved a suppressed HIV-1 RNA on a ritonavir-boosted PI regimen, with a median time to HIV-1 RNA <50 copies/mL of 182 days. By log-rank analysis, these curves were not significantly different (p=0.18).
Time to discontinuation of initial regimen
A total of 106 patients and 57 patients discontinued NNRTI and ritonavir-boosted PI therapy, respectively (Figure 2). No significant difference was found in the time on therapy before discontinuation of the regimen (log-rank test; p=0.26).
Factors associated with time to undetectable HIV-1 RNA
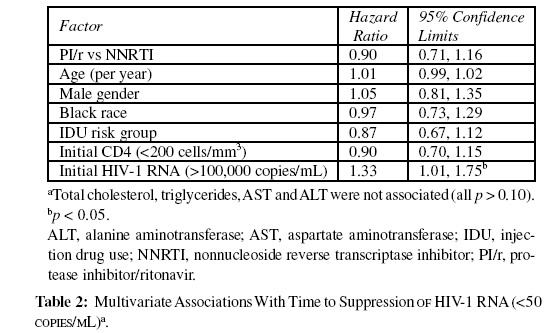
Multivariate Cox proportional hazards regression was used to determine factors associated with time to HIV-1 RNA <50 copies/mL. Only a higher baseline HIV-1 RNA was independently associated with longer time to suppression to <50 copies/ mL (p<0.05). Age, sex, race, HIV risk group, baseline CD4 cell count, ARV class (ritonavir-boosted PI vs NNRTI), and lipid or liver enzyme levels did not affect the time to undetectable HIV1- RNA level (Table 2).
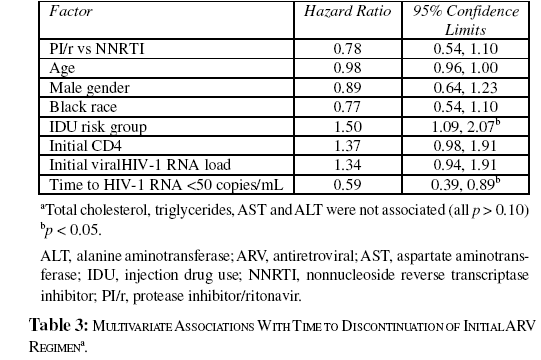
By multivariate Cox regression, a significant association was observed between injection drug use and discontinuation of the initial regimen (Table 3). Using time to HIV-1 RNA <50 copies/ mL as a time-dependent covariate in the Cox regression, there was also shown to be a significant association between a shorter time to HIV-1 RNA <50 copies/mL and length of time patients remained on the initial regimen (median time on ritonavirboosted PI=1460 days, NNRTI=1070 days; relative hazard =0.59, 95% confidence interval [CI]: 0.39, 0.89; p=0.012). No other factors were associated with discontinuation.
Time to HIV-1 RNA rebound in patients achieving undetectable HIV-1 RNA
The time to rebound in HIV-1 RNA level in patients achieving an undetectable level of HIV-1 RNA (i.e., <50 copies/mL) on their first ARV regimen was also analyzed. This analysis included 162 patients who received an NNRTI and 114 patients who received a boosted PI. As shown in Figure 3, Kaplan-Meier estimates were used to plot the time to viral rebound to >500 copies/mL beginning with the time of first HIV-1 RNA suppression to <50 copies/mL. Notably, there was a significant difference between the regimens. Those patients who received a ritonavir-boosted PI-based regimen were more likely to have virologic rebound than those patients who received an NNRTIbased regimen (log-rank test; p=0.05). However, only about 25% of patients had a virologic rebound out to 2 years after achieving virologic suppression.
Changes in laboratory parameters during antiretroviral therapy
There were no differences in the mean changes in laboratory measures of lipid and liver enzyme levels that occurred from therapy initiation to therapy switch. Lipid levels increased by a similar amount in the ritonavir-boosted PI and the NNRTI treatment groups. No significant differences were seen between the groups with regard to these measures. However, liver enzyme levels remained relatively stable over time, again with no significant differences between the two regimens.
Self-reported adherence, quality of life, and adverse events
There were also no differences in self-reported ARV adherence, QoL, and AEs at therapy initiation versus therapy switch. The mean number of reported missed doses (days on which at least 1 dose was missed, with a range of 0–3) in patients who received a ritonavir-boosted PI was twice that of patients who received an NNRTI; however, the difference was not significant. Quality of life (both overall and in terms of activities of daily living) did not appreciably change in either group over time. There was also no difference in the rate of reported AEs.
Genotypic resistance
Finally, genotypic resistance was assessed in 51 patients on an NNRTI and 31 patients on a ritonavir-boosted PI who switched therapy and had a genotype measured at the time of switching. The most common mutation, occurring in 20 NNRTI-treated patients, was the K103N mutation, which has been associated with virologic failure and broad cross-resistance to first-generation NNRTIs, such as efavirenz and nevirapine (Parienti et al., 2004; Hirsch et al., 2008). PI mutations occurred less frequently and there was no occurrence of a major mutation to the PIs used.
Our results, based on data from a clinical practice, showed that the time to achieving a suppressed HIV-1 RNA (<50 copies/ mL) was relatively long, ranging from 4.5 to 6 months (Figure 1). A total of 162 NNRTI-treated patients achieved complete virologic suppression within a median time of 140 days and 114 PI-treated patients achieved complete virologic suppression within a median time of 282 days. By log-rank analysis, these curves were not significantly different (p=0.18). Although there was no difference in the time to discontinuation of initial therapy between the groups, a shorter time to viral suppression was associated with a longer duration of effective therapy. Approximately 75% of those patients who achieved an undetectable HIV-1 RNA level maintained a suppressed HIV-1 RNA level over a two-year follow-up period, although those patients receiving an NNRTI-based regimen were more likely to maintain a suppressed HIV-1 RNA than those receiving a PIbased regimen (log-rank test; p=0.05) (Figure 3). Therefore, the impact of a shorter time to HIV-1 RNA suppression on subsequent clinical outcomes is unclear. The reason for the earlier discontinuation rate in those patients with a history of injection drug use is unknown, although active injection drug use is associated with non-adherence, and these patients self-report a higher frequency of AEs than do non-injection drug users (Carrieri et al., 2007).
These regimens were equally tolerable, with no significant differences in AEs, adherence, or QoL as self-reported by patients. Although the mean number of missed doses reported in patients receiving a ritonavir-boosted PI was twice that of patients who received an NNRTI, this difference was not significant. Metabolic alterations in lipid metabolism, similar to metabolic syndrome, have been associated with PIs in numerous studies (Powderly, 2002; Carr et al., 1998; Flint et al., 2009; Sax and Kumar, 2004; Crane et al., 2006; Fellay et al., 2001; Mary- Krause et al., 2003; Lundgren et al., 2009) and, to a lesser extent, with NNRTIs (Powderly, 2002; Shikuma et al., 2007). Yet lipids increased a similar amount in the PI and NNRTI groups. Liver enzymes, which have been reported to be elevated in patients receiving NNRTIs (Rivero et al., 2007; Kontorinis and Dieterich, 2003; Abrescia et al., 2005), remained stable in both groups.
An alternative class of drugs, the integrase inhibitors, which appears to a have shorter time to HIV-1 RNA suppression and possibly fewer AEs than PIs and NNRTIs, is now available (Steigbigel et al., 2008; Lennox et al., 2009; Markowitz et al., 2007). In the Phase III BENCHMRK trials, raltegravir, the first Food and Drug Administration-approved integrase inhibitor, demonstrated rapid viral suppression in treatment-experienced patients, with 61.8% of raltegravir-treated patients achieving complete HIV-1 RNA suppression at week 16 compared with 34.7% of patients treated with an optimized background regimen only (Steigbigel et al., 2008). Likewise, in the Phase III STARTMRK trials, which compared raltegravir to efavirenzbased therapy in treatment-naïve patients, the time to virologic response was shorter for raltegravir recipients (p<0.0001) (Lennox et al., 2009; Markowitz et al., 2007). The rapid decay observed in patients on integrase-inhibitor-containing regimens reflects an expected consequence of the fact that this drug acts later in the life cycle (Sedaghat et al., 2008). However, a specific association between earlier HIV-1 RNA suppression from an integrase inhibitor and improved clinical outcomes has yet to be shown and would need to be demonstrated before this characteristic of raltegravir could be considered a relative benefit.
The newer PIs, tipranavir and darunavir, have demonstrated more potent and durable suppression and immunologic responses, compared with the older generation of PIs in the RESIST (Hicks et al., 2006) and POWER 1 and 2 trials (Clotet et al., 2007), respectively. In addition, darunavir may have a more tolerable AE profile (Clotet et al., 2007; Capetti et al., 2009). A newer NNRTI, etravirine, may be more tolerable than efavirenz, with fewer central nervous system AEs (Madruga et al., 2007; Lazzarin et al., 2007). As these newer drugs become more frequently used in clinical practice, it will be important to determine whether these classes (and novel combinations) lead to improved outcomes.
Several factors may limit the findings of the study. First, since this is a study of actual clinical practice, HIV-1 RNA levels were not obtained at predefined intervals of time after initiation of ARV therapy. It is our standard of practice to assess HIV-1 RNA according to established guidelines, which recommend first measurement within 2 to 4 weeks of initiation and subsequent measurements every 4 to 8 weeks until HIV-1 RNA suppression is achieved (US Department of Health and Human Services, 2009). Therefore, the true time to HIV-1 RNA suppression is likely to be slightly shorter than the time that was measured. Second, generalizations cannot be extrapolated from these analyses to other populations because the patient sample was restricted to the JHHCC, which includes patients from Maryland only. Third, these analyses did not include patients on the newer generations of PIs (e.g., tipranavir and darunavir) and NNRTIs (e.g., etravirine), which were unavailable at the time of study inception. Therefore, it was not possible to assess clinical outcomes for these agents. Of note, the K103N mutation observed in 20 patients receiving NNRTIs confers cross-resistance to the earlier generation of NNRTIs used in this study but it has not been associated with etravirine resistance (Hirsch et al., 2008).
In conclusion, using the resources of the JHHCC to analyze clinical outcomes in treatment-naïve patients initiating PI- and NNRTI-based HAART regimens, it was shown that the time to achieving complete virologic suppression was relatively long, ranging from 4.5 to 6 months. Although there was no difference in the time to discontinuation of initial therapy between the groups, a shorter time to viral suppression was associated with a longer duration of effective therapy. The clinical significance of this association is unclear. Factors associated with improved outcomes may include time to HIV-1 RNA suppression for some drugs but will also include tolerability, ease of dosing, shortand long-term AEs, and the resistance profile. As newer classes of drugs become increasingly used in clinical settings, it will be important to determine whether these classes will lead to improved outcomes in clinical practice.
We would like to thank Accel Health, a division of Corbett Accel Healthcare Group New York, LLC, for their assistance with manuscript formatting and retrieving key references. RM has received support from Merck, Gilead, Pfizer, Bristol-Myers Squibb and GlaxoSmithKline.