Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Mini Review - (2022)
Nanogels (NGs) have kindled great interest in a wide variety of biomedical applications over the past few decades. This has given rise to numerous methods to synthesize these globules but often times the use of toxic additives decrease the biocompatibility of the synthesized gels. The method presented in this review uses high-energy ionizing radiation to induce the crosslinking of macromolecules in aqueous solution. This method effectively increases the biocompatibility of the NGs by eliminating the need of toxic monomers, chemical cross linkers, initiators etc. This mini-review will provide a brief discussion of the theory and methodology behind this method. Nanogels applications as related to cell therapy will also be explored.
Nanogels; Ionizing radiation; Radiation-induced synthesis nanogels; Intramolecular crosslinking
Nanogels (NGs) are a type of nanoparticle that consists of internally crosslinked macromolecules with solvent molecules (usually that of water) filling the empty spaces between the segments. Over the past decades, NGs have shown incredible promise in the biomedical field specifically as nanocarriers for drug delivery systems, cell therapy, tissue engineering, gene therapy, sensors and imaging.
Traditional synthetic methods are based on the polymerization of monomers in solution or cross-linking macromolecules with the help of cross-linking agents. However, these methods come with major toxicity concerns, which complicate the practical use of these nanoparticles in medical practice with human participants.
However, as of the late 1990s, an alternative radiation-based method for the synthesis of NGs was introduced that promises to effectively remove all toxicity concerns from the equation. In this method, high-energy low-LET (Linear Energy Transfer) ionizing radiation is used to induce crosslinking of aqueous polymers. The beauty of this method lies in its simplicity since essentially all you need is your polymer of interest dissolved in an aqueous medium.
This mini-review seeks to provide a brief overview of the discussions around the topic.
There are numerous other review papers available in the literature that cover in details important aspects surrounding this method [1,2].
Ionizing radiation
Ionizing radiation is a type of radiation that contains enough energy to ionize atoms of interest. The radiation used in this method usually consists of high energy in the form of photons (i.e. gamma rays from a Cobalt-60 source) or fast-moving particles (i.e. fast electrons from an e-beam). Upon the irradiation of an aqueous medium, the water essentially absorbs all the energy. Since the polymer is present in dilute concentrations, any direct interaction of the radiation with the polymer is negligible. Indeed the effect of radiation on the polymer is a ‘secondary’ effect that occurs at the final stages of water radiolysis. At this point the reactive species formed by the scission of water molecules, go on to abstract hydrogens from the polymeric backbone. This creates a series of radicals on the polymer that undergo crosslinking to produce the NGs. Important units when discussing radiation include dose which is the amount of energy absorbed by matter and is measured in grays, or Gy. The G-value or the radiation chemical yield which refers to the moles per absorbed energy (mol/J) of ions, radicals, or molecules produced or consumed.
Water radiolysis
The interaction of radiation and water has been a widely studied topic since the turn of the 20th century. The development of reactors in the mid-1900s greatly improved our understanding by introducing new radiation sources with higher dose rates and penetrating power. The advent of pulse radiolysis around this time further allowed scientists to study reactions down to picoseconds. Water radiolysis occurs under a second, thus the importance of greater time resolutions cannot be understated.
Energy deposition in water is highly dependent on the type of ionizing radiation in question. For low-LET radiation, energy is deposited in distinct and spaced-out regions called “spurs”, “blobs” and short-tracks. A spur is a region where the energy deposited ranges from 6-100 eV. A blob ranges from 100-500 eV and a shorttrack ranges from 500-5000 eV. The energy depositions in water are not homogeneous but once the short-lived reactive species are produced from the splitting of water, they diffuse throughout the solution quickly and react with different solutes (i.e. polymer) present.
There are three stages in water radiolysis known as the physical stage (time ranging from initiation to 10-15 s), the physio-chemical stage (10- 15 to 10-12 s) and the chemical stage (10-12 to 10-9 s). A more in-depth description of the three stages is available elsewhere [3,4].
The general reactive species formed during water radiolysis are eaq−, •OH, H•, H2O2, H2, H3O+, OH−. The •OH and H• radicals can abstract hydrogens from the polymeric backbone so are of special interest. It is possible to increase the yield of •OH by bubbling N2O throughout the solution which converts the eaq− into •OH. The general equations for water radiolysis are as follows:
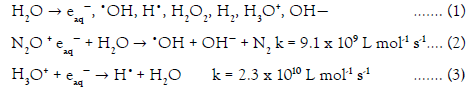
Polymer radicals
The polymer radicals are short-lived and undergo various reactions as they decay. The main reaction of interest is radical recombination reactions that form a new C-C bond. Recombination may occur inter-molecularly or intra-molecularly. To form a nanogel, intramolecular recombination must dominate over inter-molecular recombination. Intra-molecular recombination creates what is sometimes referred to as ‘loop formation’, which creates tight coils of internally crosslinked polymer networks without increasing the molecular weight of the previously linear polymer. In contrast, intermolecular recombination links together many different polymer chains which creates product with an increased molar mass. When inter-molecular recombination dominates, a larger macroscopic gel is formed. In practice, it is not possible to ever entirely avoid either inter or intra recombination. However, parameters such as initial polymer concentration, dose rate and temperature can be modified to control which of the two types of recombination reaction dominates. In general, low polymer concentrations, highdose rates and optimized temperature ranges will lead to greater intra-molecular recombination and the formation of nanogels.
Aside from recombination, the polymer radicals can also undergo degradation reactions and disproportion reactions. When the radicals undergo degradation, the polymer chain segment experiences chain scission without the loss of a radical on a carbon. Since there is no net loss of a radical during degradation, in principle it does not compete with recombination. However, if there is too much chain scission, no nanogel will form. Disproportion reactions involve the formation of a C=C double bond. This can also occur via inter or intra pathways and competes with recombination reactions. Though there is no way to eliminate these side reactions, they can be minimized by changing certain parameters.
Product analysis
Once the nanogels have been synthesized by some form of low-LET radiation, the reaction progress and formation can be followed by a variety of different techniques. The Radius of gyration (Rg) is a very important characteristic feature that is almost always studied to show the formation of a NG. A decrease in this radius shows that the product has formed tighter coils a strong indication of intra-molecular crosslinks. Multi-angle static laser light scattering technique, MALLS, is often used as it can measure the Rg and weight-average molecular weight simultaneously. An increase in molecular weight without much change in the Rg would indicate the domination of inter-molecular crosslinking and that macro-gel (rather than a nano-gel) was formed.
Viscometry is another technique that is used to study the final products. If there is an increase in the compactness of the polymer coils due to the formation of NGs, there will be a significant decrease in the viscosity of our product.
Asymmetric Flow Field Flow Fractionation (AFFFF or AF4) is relatively newer technique that has become popularized in recent types to characterize and separate nanoparticles by size. Visualization of individual nanogels is often conducted using AFM and SEM.
Applications of nanogels in cells
Many studies can be found in the literature where scientists have used this technique to synthesize nanogels that were found to be useful in many biomedical applications including in the treatment of Alzheimer’s disease and tumour cell targeting [5,6].
It was shown that a controlled release of biological molecules in response to cancer cells was Possible With Carboxyl-Functionalized Polyvinylpyrrolidone (PVP-co-acrylic acid) nanogels synthesized by e-beam irradiation [6]. The technique relied on the NGs responding to the over-exposed proteins on tumour cells differently than normal cells. These targeted approaches reduce the adversary side effects of random cancer drug distribution in the body and simultaneously improve the drug efficiency as the NGs can carry large amounts of cargo.
Elsewhere, other nanogel systems have demonstrated that nanogel carriers can effectively deliver antisense Oligonucleotides (ODN) across the Blood-Brain Barrier (BBB), a task that is relatively difficult to achieve with macromolecules [7]. The ODN was transported through the brain micro vessel cells following the transcellular pathway. The ODN remained intact in the nanogel, showing little degradation when compared to free ODN.
Nanogels are also very effective in intracellular uptake particularly due to their small size, relatively high degree of uptake of therapeutic agents and low toxicity. A self-assembled system of Heparin-Pluronic (HP) nanogels was prepared and shown to be a very effective vehicle for the intracellular delivery of the protein Ribonuclease A (RNase A) [8]. The HP nanogel formed a stable nano-sized lump that had a gel-like structure with a high affinity for protein and a loading efficiency around 80%-99%. The uptake mechanism was reported to be via caveolae/lipid-raft-mediated endocytosis. The RNase A loaded NG saw a significant decrease in the hydrodynamic size most likely a result of the binding between RNase A and heparin. This study is just one example of how NGs can enhance the delivery of this anticancer therapeutics into HeLa cells.
In this short overview, we have attempted to outline some of the main components regarding the synthesis of nanogels via ionizing radiation and applications of these particles in cellular systems particularly related to drug delivery. NGs have a variety of other application as carriers or cell markers for medical testing, blocking agents for dental channels, chemical/biological sensors, etc.
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
Citation: Ashfaq A, Ulanski P, Al-Sheikhly M (2022) Overview of Radiation-induced Crosslinking of Macromolecules to Synthesize Nanogels for Biomedical Applications. J Cell Sci Therapy. S10:351.
Received: 14-Apr-2022, Manuscript No. JCEST-22-15479; Editor assigned: 18-Apr-2022, Pre QC No. JCEST-22-15479(PQ); Reviewed: 02-May-2022, QC No. JCEST-22-15479; Revised: 09-May-2022, Manuscript No. JCEST-22-15479(R); Published: 16-May-2022 , DOI: 10.35248/2157-7013.22.S10.351
Copyright: © 2022 Ashfaq A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.