Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Review - (2023)Volume 11, Issue 6
Oxidative stress causes harmful effects on normal cells in the human body. However, leukemia cells gain resistance to oxidative stress’s harmful effects through heightened glycolysis, increased activity of the Pentose Phosphate Pathway (PPP) and modified mitochondria. These systems potentially lead to leukemia cell resistance via the antioxidant effects of the glycolysis products and redox signaling pathways overexpressed in leukemia cells mitochondria. Anthracycline agents used as a chemotherapeutic drug can cause significant amounts of oxidative stress to overcome the anti-oxidative stress capability of leukemia cells, leading to the induction of severe damage and apoptosis in these cells. Apoptosis in leukemia cells can also result through the help of Ataxia Telangiectasia Mutated (ATM) signaling pathways. Throughout this review paper, we will discuss how leukemia cells can efficiently utilize their mechanisms to thrive in heightened oxidative stress-induced environments. Then we will examine the role of chemotherapy-induced extreme oxidative stress leading to leukemia cells demise while highlighting key points of further advancement in treatment of leukemia disease.
Oxidative stress; Pentose phosphate pathway; Leukemia; Ataxia telangiectasia mutated signaling pathways
Leukemia, a highly prevalent cancer, originating from abnormal white blood cells that tarnish the bone marrow and is refractory due to the existence of leukemia cells. Estimated 60,000 cases in 2023, around 38% of which end in death. Leukemia comes in a variety of forms, mainly including acute myeloid leukemia, chronic myeloid leukemia and their variants [1-3]. Oxidative stress is known to play a critical role in the pathogenesis of cancer diseases such as leukemia. Due to numerous mechanisms that contribute to leukemia cell’s resistance to oxidative stress, oxidative stress doesn’t harm leukemia cells to the same degree as normal cells. Due to the advanced mechanisms of leukemia’s cellular structure, the cell maintains resistance to oxidative stress [4]. Leukemia cell use of glycolysis, Serine/threonineprotein phosphatase 2A, mammalian Target of Rapamycin Complex 1 (mTORC1), Pentose Phosphate (PPP) Pathway, AMPActivated Protein Kinase (AMPK), Sirtuin2 (SIRT2), Nuclear factor erythroid ATN 2–related factor 2(Nrf2), and the Voltage- Dependent Anion Channel (VDAC) is known to contribute to cancer cells protection against oxidative stress [5,6].
Nevertheless, by utilizing chemotherapy drug treatment, scientists have developed ways to induce severe oxidative stress within leukemia cells. Chemotherapy, a common therapeutic approach for leukemia, produces severe oxidative stress and is able to reduce leukemia cell’s ability to resist oxidative stress through mitochondrial damage, DNA damage, and other cellular mechanisms [7]. Leukemia cells are able to develop a resistance to oxidative stress due to their molecular structure, though they can be retreated from extreme oxidative stress generated through chemotherapy’s mitochondrial damage, DNA damage, and more [8]. This is vital for cancer research and targeting treatment in the cells. Although extensive research has been conducted to make progress towards a cure for leukemia, understanding the connection between leukemia cells and oxidative stress can further our knowledge in cancer research and thereby the treatment [9- 12].
Oxidative stress occurs due to an imbalance between free radicals and antioxidants. Free radicals are a class of unstable molecular species that contain unpaired electrons. Free radicals have been proven to be beneficial to the body, as small amounts can regulate cell proliferation and function as a defense system against pathogenic diseases as long as overproduction doesn't occur [13]. Free radicals come in many forms, but those associated with oxidative stress are known as Reactive Oxygen Species (ROS). The main types of ROS to highlight are hydrogen peroxide and superoxide, which are abundant in the majority of tumor cells. The Electron Transport Chain (ETC) in cells generates superoxide and hydrogen peroxide primarily through the mitochondria [14]. Production of ROS transpires from enzymatic and nonenzymatic sources (Pizzino). The most prevalent source of enzymatic activity is NADPH oxidase (NOX), found in the mitochondria. Although the electron transport chain is a major generator of ROS, the family of NOXs is also a big source of ROS [15]. Non-enzymatic sources of oxidative stress include mitochondrial respiration and cell exposure to ionizing radiation (Pizzino). Antioxidants are created to detoxify free radicals and achieve a balance between the two in order to regulate the amount of free radicals produced [16,17]. Antioxidant enzyme systems produce antioxidants to combat ROS. Catalase and Superoxide Dismutase (SOD) systems, for example, are found in leukemia cells. Superoxide dismutases function to break down hydrogen peroxide, while Catalases work to convert hydrogen peroxide to water. If an imbalance is created with more free radicals than antioxidants, oxidative stress occurs. If an excessive buildup of ROS occurs, cell injury and death can occur [18-20]. Paradoxically cancer cells, due to their cell structure and modification, are able to have protection against oxidative stress. Though present in cancer cells, if permanently raised, the levels of ROS can inhibit their resistance to oxidative stress and lead to cell death [21].
Leukemia cells resistance in correlation to their mitochondria is derived from how they utilize glycolysis. Glycolysis is the breakdown of glucose in the cytoplasm and the initial step in cellular respiration. A single glucose molecule is phosphorylated by the enzyme hexokinase to form Glucose-6-Phosphate (G6P) [22]. From here, depending on the cell, the course can be direct towards the PPP pathway. If not diverted to the PPP pathway, G6P is then converted into fructose 6-phosphate. Then the enzyme Phosphofructokinase directs fructose 6-phosphate into fructose 1,6-bisphosphate which eventually leads to the production of pyruvate [23]. Glycolysis can function anaerobically and aerobically depending on the presence of oxygen. Recognized as the Warburg effect, leukemia cells are more likely to operate glycolysis aerobically than using oxidative phosphorylation, where fermentation, resulting in a byproduct of lactase, occurs more often despite oxygen present. Favored by leukemia cells due to its role in increased glucose uptake leading to more products of the PPP pathway [24]. ATP production is heavily relied on by leukemia cells as they use the majority of their energy supply for metastasis, the creation of additional malignant cells away from the initial site of cancer, supporting their reliance on glycolysis [25]. Usually aerobically glycolysis is an insufficient way to produce ATP compared to other methods; however cancer cells, such as leukemia, prefer due to the increased rate of ATP production. In addition, the large amount of lactase, high in carbon, exerted during glycolysis doesn’t go to waste, but instead causes rapid incorporation of carbon resulting in fast cell division. Through The Warburg effect, it creates a source of carbon, supporting cell growth and division (Figure 1) [26].
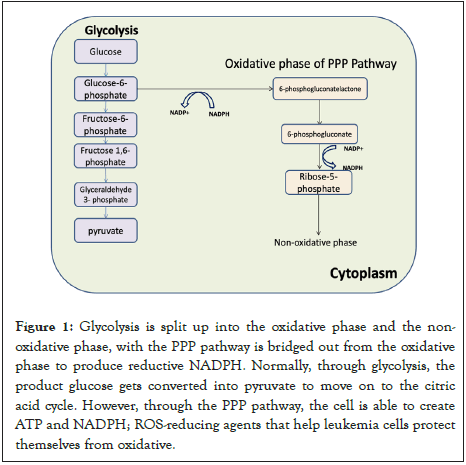
Figure 1: Glycolysis is split up into the oxidative phase and the nonoxidative phase, with the PPP pathway is bridged out from the oxidative phase to produce reductive NADPH. Normally, through glycolysis, the product glucose gets converted into pyruvate to move on to the citric acid cycle. However, through the PPP pathway, the cell is able to create ATP and NADPH; ROS-reducing agents that help leukemia cells protect themselves from oxidative.
The use of glycolysis can differ in leukemia due to differences in cell structure in order to become more glycolic. For example, in B-Cell Acute Lymphoblastic Leukemia (B-ALL), when G6P is formed and the pathways can be pushed towards the PPP pathway, B-cell leukemia cells are able to use Serine/Threonine- Protein Phosphatase 2A (PP2A), involved in redirected glycolysis towards the PPP pathway, to their advantage [27]. PP2A functions as a tumor suppressor; thus, it is inactivated in the majority of leukemia cells, with the exception of B-Cell Acute Lymphoblastic Leukemia (B-ALL). B-ALL relies on PP2A for survival and to move glucose from glycolysis to utilize the PPP pathways. Furthermore, BCR-ABL mutations, which act as fusion oncogenes, support Chronic Myeloid Leukemia (CML) cells use of glycolysis by speeding up the transfer of glucose for faster breakdown and ATP production [28-30]. The increase in glycolysis results in an increase in the use of the Pentose Phosphate (PPP) Pathway, which produces NADPH. As an electron carrier, NADPH molecules will donate their electrons to antioxidants in order for them to keep up with the overproduction of ROS and maintain a balance between antioxidants and free radicals more use of glycolysis, more NADPH, and less oxidative stress [31,32].
Glycolysis is associated with the Pentose Phosphate Pathway (PPP), an antioxidant pathway with two phases involved in the production of NADPH and the oxidation of glucose. The oxidative phase is critical to leukemia cells due to its production of 2 NADPH per glucose molecule through dehydration and oxidative decarboxylation [33]. NADPH is produced through oxidative stress, and R5P is produced through the non-oxidative phase, which is important to many bio macromolecules. Due to leukemia cells extensive use of glycolysis, they regulate the phases of the vital pathways based on the product they need most [34,35]. If NADPH is needed, R5P will be converted into G3P and F3P, products used to make G6P, which goes through the oxidative phase to produce more NADPH. This commonly occurs when the presence of oxidative stress increases. High activity of G6PH will increase the PPP flux, thereby increasing the products of the pathway [36]. Cancer cells achieve this by stimulating specific oncogenic pathways, such as Ras and Src. In CML and AML cells, the SIRT2 gene expression was shown to be overexpressed through recent studies. The gene produces sirtuin 2 and removes an acetyl functional group to promote NADPH production [37]. This increases G6PH activity. In the PPP pathway, G6PDH is an important component in how the enzyme is regulated to produce NADPH by the NADP+/NADPH ratio and provide stability to cancer cells [38]. In addition to the production of NADPH molecules, the PPP pathway also produces pentose phosphate for nucleic acid synthesis and cell survival. Specific transcription factors, such as d (EV1), can contribute to leukemia cell’s reliance on the PPP pathway. EVI1 is very important for leukemia cell development (Figure 2). When EVI1 is overexpressed, the enzyme hexokinase 3 is upregulated (Mizuno). As a result of the pathway's importance to cancer cells, potential therapeutic targets include PPP pathway inhibitors. cyclic AMP (c-AMP) reduces the expression of G6PDH in some leukemia cells. It suppresses G6PDH, which reduces NADPH levels and reduces leukemia cell’s resilience to oxidative stress [39-40].
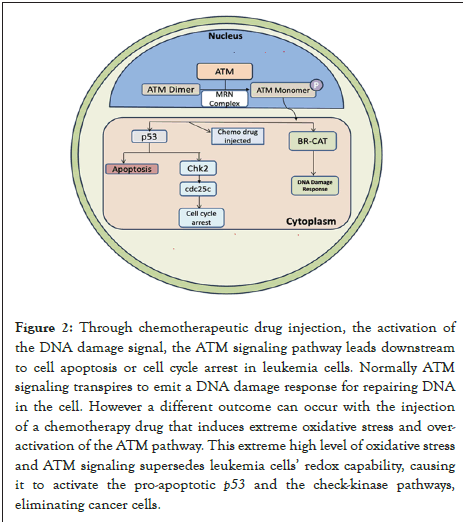
Figure 2: Through chemotherapeutic drug injection, the activation of
the DNA damage signal, the ATM signaling pathway leads downstream
to cell apoptosis or cell cycle arrest in leukemia cells. Normally ATM
signaling transpires to emit a DNA damage response for repairing DNA
in the cell. However a different outcome can occur with the injection
of a chemotherapy drug that induces extreme oxidative stress and overactivation
of the ATM pathway. This extreme high level of oxidative stress
and ATM signaling supersedes leukemia cells’ redox capability, causing
it to activate the pro-apoptotic p53 and the check-kinase pathways,
eliminating cancer cells.
Understanding leukemia cell molecular mechanisms will help us understand their adaptive mechanisms in regards to glycolysis that aid them in developing resistance to oxidative stress. Leukemia cells have proven to use downstream signaling pathways to contribute to their glycolic nature [41]. A common one they rely on is mTORC1, complex 1 of the signaling pathway mTOR, which is highly activated in Acute Myeloid Leukemia (AML). The pathway receives signals regarding growth and energy supply to induce cell growth. A previous study has shown that when mTORC1 is inhibited in inhibited AML cells; glucose intake is reduced, linking the pathway to supporting leukemia cells dependence on mTORC1 in glycolysis by the cancer cell. mTOR is initiated once P13K is activated in the presence of extreme oxidative stress, inducing the expression of genes related to glycolysis and the PPP pathway, resulting in increased glucose consumption [42]. mTORC1 supports leukemia cell’s extreme use of glycolysis, resulting in increased glucose flux through the PPP pathway and increased NADPH. Leukemia cells reliance on mTOR can influence further advancement in mTORC1/ mTORC inhibitors [43].
Leukemia cells also rely on AMP-Activated Protein Kinase (AMPK) to subdue ROS production. AMPK is essential for leukemia development when activated in response to a decrease in ATP in leukemia cells. AMPK is able to increase glycolysis production through 6-Phosphofructo-2-Kinase (PFK2) [44]. PFK2 is an enzyme associated with the regulation of glycolysis. When glucose presence increases, insulin increases the levels of PRK2, boosting glycolic activity. This increases the NADPH levels generated via the PPP pathway, strengthening leukemia cell’s resistance to oxidative stress. Understanding the role of AMPK and many other aspects of leukemia cells provides insights into potential treatments for this disease. Understanding leukemia cell’s adaptive mechanisms, such as AMPK, opens potential inhibitors for the P13K signaling pathway to reduce their protection from oxidative stress and lead to their demise. Other components of leukemia's cellular structure can explain their resistance to oxidative stress and provide further advancements in treatment for the disease [45].
Mitochondria are vital for cell survival and are commonly known for their role in ATP production through oxidative phosphorylation or glycolysis. The organelle contains mDNA to produce proteins to carry out processes. Components of oxidative phosphorylation include the Electron Transport Chain (ETC), critical for ATP production as well as a regulator for cell death through ROS [46]. ETC is composed of five complexes made of various proteins transcribed from mDNA, through which electrons are passed to release energy in the form of ATP. That energy release forms a proton gradient, which is used to produce an abundance of ATP through membrane protein ATP synthesis. The receiving molecule of the ETC is oxygen converted into water; however, 0.2%-2% of electrons in the ETC leak out and interact with oxygen to produce ROS [47]. This more commonly occurs in cancer cells than in normal cells. Production of ROS mainly occurs in complexes 1 and 3, where NADH is oxidized, and reducing ubiquinone to ubiquinol, and electrons are taken through the intermembrane space up to complex 4. In complex 1, electrons from NADH to CoQ produce ROS. The Electron Transport Chain (ETC) functions as a substantial source of ROS through the mitochondrial respiratory chain and NADPHoxidase. TCA is selectively altered in cancer cells to promote cell proliferation through citrate synthase, an enzyme involved in the initial steps of TCA [48]. Because the ETC is a source of ROS, cancer develops mitochondrial changes that protect the cell from oxidative stress. Systems such as the VDAC and signaling systems work to the cancer cells advantage when dealing with oxidative stress. It induces a change in the cancer cell’s metabolic reprogramming to produce a more glycolytic cell, elevating cancer cells’ dependency on glycolysis. Components of cancer cells’ mitochondria are modified to serve as protection against oxidative stress. VDAC is a signaling system within the outer membrane of the mitochondria that regulates what metabolites enter and exit the mitochondria and contributes to cellular glycolysis dependency by assisting cancer cells [49]. Among the metabolites that pass through, AMP can pass through VDAC, creating an ATP/AMP ratio. This regulation regulates the activation of AMPK. Due to AMPK's role in glycolysis within cancer cells, the VDAC pathway supports the production of ATP and NADPH through the PPP pathway in glycolysis. VDAC operates either on an open phase or an off phase, which determines the cytosolic ATP/ADP ratio, and is also altered in cancer cells, influencing cancer progression and metabolism due to its role in ATP production. When the signaling pathway is turned on, it increases the function of mitochondrial metabolism, which functions more efficiently compared to normal cells [50]. Furthermore, VDAC interacts with the enzyme Hexokinase 2 (HK2), which is involved with G6PH to control the use of ATP in cancer cells, implying that VDAC also has a connection to glycolysis. Interestingly, VDAC can also trigger mitochondrial death via the mitophagy process in order to alleviate oxidative stress. Cancer cells secrete a survival protein called Nrf2 for protection against oxidative stress. Once oxidative stress becomes present in the cell, Nrf2 is produced from the mitochondria. Studies have previously shown that through genes such as p53, Nrf2 is overexpressed in leukemia cells, specifically Acute Myeloid Leukemia (AML). As ROS begins to develop in the mitochondria, Nrf2 leads to the expression of genes that are involved in maintaining equilibrium. When it is overexpressed, it results in the production of ROSscavenging enzymes that help with metabolic reprogramming and maintain the balance between antioxidants and ROS.
Chemotherapy is a popular treatment for all types of cancers, including leukemia. Chemotherapy consists of over 100 drugs designed to target different moments of the cell cycle. The drugs are commonly injected through the vein, targeting the genes in the nucleus of the cancer cell. However, in the process, chemo drugs can also destroy normal cells, resulting in painful side effects and weakening the patient’s immune system.
In addition to chemo drugs targeting cancer cell genes, they can induce apoptosis in cancer cells through oxidative stress despite leukemia cells resistance.
All antibiotics found in chemotherapy drugs generate ROS; however, anthracyclines produce the most compared to others. Doxorubicin (DOX), daunorubicin, and epirubicin are a few examples, with this paper focusing on doxorubicin. DOX is efficient in decreasing leukemia DNA transcription by inhibiting topoisomerase 2 to prevent DNA strands from unwinding, preventing DNA replication and cancer cells growth and development. Additionally, DOX stops the growth of tumors by accessing membrane proteins such as CREB3L through regulated intramembrane proteolysis. Previous studies have demonstrated that when CREB3L1 splits, an amino-terminal domain is released in the cytoplasm, initiating the production of genes linked with cellular proliferation inhibition. When CREB3L1 is overexpressed, it allows tumor cells to become more susceptible to doxorubicin. Moreover, DOX is able to produce ROS through interruption of the ETC in mitochondria, where quinone is reduced to semiquinone. Electrons are brought to oxygen by NADH dehydrogenase, which produces superoxide. Furthermore, due to the ETC in the mitochondria, oxidative stress targets DNA damage to the cell’s mitochondrial DNA (mDNA) due to the mitochondria being a primary source and target for oxidative stress. As more damage occurs, mitochondria become more vulnerable to oxidative stress due to the lack of protection, leading to apoptosis. Due to their drive to multiply and spread, cancer cells often replicate their DNA, which can result in further DNA mutations. Extreme oxidative stress can lead to death in ROS-induced leukemia models, especially AML and CML. ROS interacts with the inner mitochondrial membrane to release cytochrome c. Cytochrome c is a mitochondrial protein that is released in the cytoplasm through an apoptotic stimulus. Once cytochrome C enters the cytoplasm, it forms an apoptosome, activating caspase 9 and achieving apoptosis of the cell. Antibiotics in chemotherapy drugs are able to activate systems in leukemia cells to produce an overwhelming amount of oxidative stress, resulting in their demise despite their resistance to oxidative stress.
Ataxia Telangiectasia Mutated (ATM) signaling pathways are tightly regulated in all cells but can function as a treatment target. They belong to the PI3K-related protein kinase family and function as a serine/threonine kinase. The ATM pathway begins by eliciting a DNA damage response at DNA lesion sites through the Mre11/Rad50/Nbs1 (MRN) complex. Once coupled to this complex, ATM becomes active and uses the kinase produced to downregulate processes vital to DNA repair, apoptosis, etc. This enables ATM monomers to be sent to the sites of DNA damage. There are multiple posterior targets that ATM phosphorylates, including BRCA1, to provoke a DNA damage response in leukemia cells. In addition to a DNA damage response, the ATM pathway can result in apoptosis or damage to the cell cycle if directed down a different path [51]. If an abundance of DNA damage is present, ATM signaling has no choice but to slip into G0 or apoptosis to save the cell. Too much oxidative stress generated by chemotherapy drugs can generate a lavish amount of oxidative stress to lead leukemia cells to respond with a DNA damage response, resulting in cancer cell apoptosis despite their developmental protection from oxidative stress. The significant amount of DNA damage forces ATM signaling to downstream check kinase 2 to cause cell cycle arrest [52]. Furthermore, the presence of oxidative stress, in addition to DNA damage, can induce ATM to become activated even without the MRN complex. ATM is initially inactivated as a dimer but is activated by establishing bonds. Oxidative stress leads to ATM signaling; the oxidative stress leads to DNA damage; it's too much for the cancer cell's ATM to handle, and it resorts to the stopping of the cell cycle to prevent division or apoptosis through kinases [53].
This review demonstrates how leukemia cells develop resistance to oxidative stress, which can be combated through extreme oxidative stress induced by chemotherapy drugs. Leukemia cell’s advanced mechanisms of glycolysis and other components of the mitochondria support their resistance to oxidative stress. Despite the different types of leukemia varying in cell type, all use glycolysis anaerobically as well as the PPP pathway. In comparison to normal cells, leukemia cells efficiently regulate mTORC and AMPK in order to become more glycolic. This applies to the PPP pathway as well. Transcription factors such as EVI1 and, moreover, G6PH are elevated in leukemia cells, which assist them in the use of the PPP pathway. Leukemia cells mitochondria are modified to provide protection against oxidative stress. ETC is a main source of oxidative stress, but leukemia cell’s VDAC signaling system plays a big role in ATP production and G6PH, which allows the cell to function more efficiently and make more use of the NADPH enzyme. Chemotherapy’s purpose is to injure cancer cells by damaging different parts of the cell. ROS-induced DNA damages chemotherapy itself. Furthermore, the paper discusses the comprehension of multiple chemotherapy drugs and cellular oxidative stress’s role in chemotherapy involving ATM signaling, DNA damage, or other cellular mechanisms. through which, high amounts of oxidative stress are created. The points covered in this article can contribute to target treatment through the use of inhibitors. Inhibitors of glycolysis can make leukemic cells more susceptible to oxidative stress. Inhibitors of the PPP can be introduced as well. Through the evidence provided, multiple treatments can be developed to diminish the incidence of leukemia as well as other cancers.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Badugu V (2023) Oxidative Stress is the Other Side of the Coin in Normal Versus Cancer Cells. J Leuk. 11:349.
Received: 23-Sep-2023, Manuscript No. JLU-23-27148; Editor assigned: 26-Sep-2023, Pre QC No. JLU-23-27148 (PQ); Reviewed: 20-Oct-2023, QC No. JLU-23-27148; Revised: 27-Oct-2023, Manuscript No. JLU-23-27148 (R); Published: 03-Nov-2023 , DOI: 10.35248/2329-6917.23.11.349
Copyright: © 2023 Badugu V. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.